Guidance on health and identification marks
Guidance on health and identification marks that must be applied to products of animal origin (POAO) such as meat, egg products, fish, cheese and milk.
The following guidance is for enforcement authorities and UK food businesses that produce POAO in the UK (Great Britain and Northern Ireland). It outlines the identification mark requirements that will allow POAO produced by UK businesses to be placed on Great Britain, Northern Ireland, EU and non-EU Markets.
What are health and identification marks?
Health marks are applied directly to the carcases of domestic ungulates and farmed game mammals (except lagomorphs) by the Competent Authority (CA) in slaughterhouses and game handling establishments. In this instance, the Food Standards Agency is the CA, as these establishments require veterinary oversight for the delivery of official controls in England, Wales and Northern Ireland. It shows that the product has been produced in accordance with food safety regulations. Food Standards Scotland (FSS) has a similar responsibility in Scotland.
Identification marks are applied to POAO by food businesses, demonstrating that it has been produced in an approved establishment and in accordance with food safety and hygiene regulations. It is typically applied to wrapping, packaging, or labelling which contains, or is attached to, the POAO.
Further down this page you can find:
- a description of the identification marks, depending on whether the food business is based in Northern Ireland or Great Britain - the UK Government recommends use of the full country code 'United Kingdom' where it is practical
- information setting out the requirements for different markets - businesses need to ensure that they use the correct identification mark depending on the target market for their POAO
Current valid identification marks
There is no minimum or maximum size for the identification mark. However, it must be legible and indelible oval mark, and the characters easily decipherable.
On the Great Britain market
The identification mark must contain either the full country name 'United Kingdom' or the 'GB' or 'UK' abbreviation for POAO produced in England, Scotland and Wales. The UK Government recommends use of the full country code 'United Kingdom' where it is practical. Businesses that want to move their POAO outside of the GB market should also check the ID mark requirement for their target market.
On the Northern Ireland market
For POAO produced in Northern Ireland, the identification mark must contain either the full country name 'United Kingdom (Northern Ireland)' or the 'UK (NI)' abbreviation followed by the approval number of he establishment. It must also contain the letters 'EC' (until 31 December 2028) or 'EU' after the approval number. FSA recommends starting the transition to the new format as per the amendment to Regulation (EC) No 853/2004 of 9 May 2024 as soon as possible to avoid surplus stock with 'EC' that would not be valid after 31 December 2028.
Moving products of animal origin from Great Britain to Northern Ireland
Legally compliant identification marks and the markets they apply to can be found in the table below.
From 1 October 2023, under the Windsor Framework, the Northern Ireland Retail Movement Scheme (NIRMS) replaced the Scheme for the Temporary Agrifood Movements to Northern Ireland (STANMI). The new scheme allows a broader range of traders such as retailers, wholesalers, caterers, and those providing food to public institutions such as schools and hospitals to move pre-packed agrifood goods which are destined for final consumers in NI. The arrangements enable consignments to move based on a single certificate, without routine physical checks. Consignments of pre-packed agrifood goods, moved from GB to NI via NIRMS can now meet GB standards in public health, marketing (including labelling) and organics.
More detailed guidance can be found on the following webpages:
- Moving Products of Animal Origin and Animal By-Products - Defra
- Northern Ireland Retail Movement Scheme: how the scheme will work - Defra
Use of identification marks on the Great Britain, Northern Ireland, EU and non-EU markets
Identification marks that must be applied and destination market.
Please note that since 9 May 2024, NI businesses in line with Regulation (EC) No 853/2004 can also use the suffix 'EU' instead of 'EC'. The suffix 'EC' will remain valid until 31 December 2028.
| Identification mark | UK region where mark is applied | Great Britain market | Northern Ireland | EU 27 market | Non-EU market |
|---|---|---|---|---|---|

|
Northern Ireland (FSA approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (FSA approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (FSA approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (FSA approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (District Council approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (District Council approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (District Council approvals) | Yes | Yes | Yes | Yes |

|
Northern Ireland (District Council approvals) | Yes | Yes | Yes | Yes |
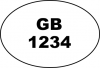
|
Great Britain (FSA approvals) | Yes | Yes | Yes | Yes |

|
Great Britain (FSA approvals) | Yes | Yes | Yes | Yes |

|
Great Britain (FSA approvals) | Yes | No | No | Yes |

|
Great Britain (local authority approvals) | Yes | Yes | Yes | Yes |

|
Great Britain (local authority approvals) | Yes | Yes | Yes | Yes |

|
Great Britain (local authority approvals) | Yes | No | No | Yes |
Revision log
Published: 30 July 2025
Last updated: 4 September 2025