Chapter 2.1 Food Chain Information (FCI) and Collection and Communication of Inspection Results (CCIR)
This chapter details the use of FCI and the collection and communication of inspection results for stakeholders.
Sections
1. Introduction
In this section
1.1 Purpose of FCI and CCIR
1.1.1 Purpose of food chain information (FCI)
Food Chain Information (FCI) should be used by slaughterhouse Food Business Operators (FBOs) to assess any potential hazards presented by the animals intended for slaughter as part of their Hazard Analysis and Critical Control Point (HACCP)-based food safety management systems.
FBOs should act upon the information provided in the FCI by making decisions about accepting animals and any special processing arrangements, for example:
- slaughter at the end of a run
- additional dressing requirements
- reduced line speed
This helps to ensure that certain veterinary medicines or animals affected by disease do not enter the food chain.
Information that must be confirmed on the FCI declaration includes:
- health status of the farm - that the holding is not under any movement restrictions for animal disease or public health reasons
- withdrawal periods have been observed - that there are no known veterinary medicine residues in the meat
- the animal's health status - that the animal to be slaughtered has not been exposed and does not show any signs of disease that may affect the safety of the meat
FCI is required for every animal intended for human consumption. The producer must provide FCI to the FBO for all animals presented for slaughter.
It is the FBO's responsibility to evaluate the FCI and then make it available to the OV without delay.
The OV must review the FCI before ante-mortem inspection to determine the inspection procedures required. It is also the OV’s responsibility to verify that the FBO's HACCP plan includes and assesses all potential hazards contained in the FCI in line with the HACCP principles and that the HACCP established procedures are correctly implemented.
1.1.2 Veterinary Attestation Number (VAN)
From 13 December 2023, all livestock farmers who produce livestock or livestock products destined for the food chain, and which may be exported to the European Union, will require proof of an annual veterinary visit.
This requirement can be fulfilled in several ways:
- proof of participation in a Defra qualifying assurance scheme, such as Red Tractor, Quality Meat Scotland (QMS), Farm Assured Welsh Livestock (FAWL), RSPCA, Lion Quality or Poultry Health Scheme. The Food Business Operator (FBO) will verify membership details (no VAN required). The assurance schemes operate their own audit and compliance processes which provide the level of confidence that assurance scheme members are meeting the requirement and therefore the VAN is not required
- in England, if a farm has had an annual visit as part of the Defra Animal Health and Welfare Review Pathway Scheme, then this visit will fulfil the requirement. The visiting vet will fill in the Pathway form and provide the VAN of the veterinary visit
- if the farm is neither part of a recognised farm assurance scheme nor receives a Pathway vet visit (for England only), then a visit must be organised with a private veterinarian and an attestation is required from the veterinarian stating the visit has taken place. A VAN number will be issued by the attending veterinarian
The vet visit will review the farm and all its livestock species for signs of notifiable diseases and biosecurity risks. The vet will generate a 20-digit Veterinary Attestation Number (VAN), which must be included on movement licences and the FCI. It will comprise of:
- the visiting vet’s RCVS number
- the County Parish Holding (CPH) number of the establishment visited
- the date of validity of the declaration
For example, 1234567 [MRCVS number] 12/345/6789 [CPH number] 0624 [Valid to the end of June 2024].
This requirement applies to farm-to-slaughter and farm-to-processor movements only (for example, animal markets); farm-to-farm movements are not affected.
If the farm is not a member of the qualifying assurance scheme or a VAN is not provided, the OV at the slaughterhouse will not be able to sign a Support Health Attestation (SHA) facilitating products derived from the animals on that consignment to be exported to the EU.
Even when meat from animals is not intended for export to the EU, there is a high likelihood that some of the animal products or by-products derived from them may be included in exports to the EU. Therefore, Defra is strongly recommending that all farm businesses ensure a veterinary visit has taken place at their farm.
Note: A 4-month implementation period starting from 13 December 2023 has been agreed with RCVS, to monitor compliance with the requirement for the Veterinary Health Attestation visits and to enable verification and addressing of any potential issues. During the implementation period farmer self-declarations can still be accepted at the abattoir, but OVs should encourage FBOs to communicate back to their suppliers and highlight that the implementation period will end on 12 April 2024 and from 13 April 2024, if there is no VAN or other evidence that a farm visit has been carried out, the OVs will not issue an SHA.
References:
- Regulation (EU) 2016/429, Article 8
- Regulation (EU) 2020/692
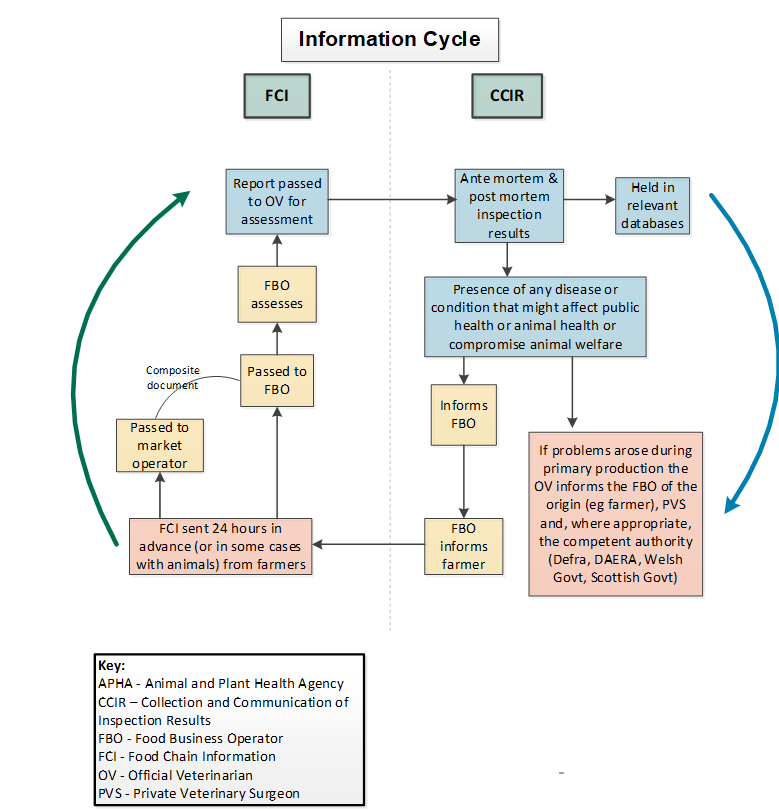
1.1.3 Purpose of collection and communication of inspection results (CCIR)
CCIR is information provided to the producer to initiate any actions required on the farm to improve animal health, animal welfare and subsequently, food safety.
Where inspection procedures reveal animal health or welfare problems that have arisen at primary production, the FSA must report direct to the producer.
Where inspection procedures reveal the presence of any disease or condition that might affect public or animal health or indicate compromised animal welfare, the OV should inform the slaughterhouse FBO.
1.2 Information cycle (FCI and CCIR)
1.3 Legislation
1.3.1 Regulations
The information cycle (FCI and CCIR) is required by Regulations (EC) 852/2004, (EC) 853/2004 and (EU) 2017/625.
Information cycle table
| Regulation | Requirement | Responsibility |
|---|---|---|
| (EC) 852/2004 | Lays down the records which FBOs rearing animals are required to keep. | FBOs for the holding of provenance (farmer or producer) |
| (EC) 853/2004 | Describes the FCI that FBOs must request, receive and act upon. | Slaughterhouse FBOs |
| (EU) 2019/624 and (EU) 2019/627 |
Requires the OV to check and analyse the FCI and to take account of this when carrying out ante and post-mortem inspections. Requires the OV to provide data from the ante- and post-mortem inspections to the slaughterhouse FBO and back to the farmer/producer when the inspections reveal the presence of any disease or condition that might affect public or animal health, or compromise animal welfare. |
OV |
1.3.2 FCI implementing measures
Regulation (EC) 853/2004 establishes that slaughterhouse operators must not accept animals onto the slaughterhouse premises unless they have requested, and been provided with, relevant FCI.
Commission Implementing Regulation (EU) 2019/627, requires the Competent Authority (CA) to inform the FBO at the holding of provenance of the minimum elements of FCI to be supplied to the slaughterhouse.
References:
- (EC) 853/2004, Annex II, Section III
- (EU) 2019/627, Article 9, Paragraph 1
1.3.3 Additional FCI requirements: broilers
Council Directive 2007/43/EC lays down the minimum rules for the protection of chickens kept for meat production.
The Welfare of Farmed Animals (Amendment) Regulations 2010 (England / Wales) implement Council Directive 2007/43/EC and specify additional Food Chain Information requirements in respect of conventionally reared meat chickens. This regulation requires that the daily mortality rate and cumulative daily mortality rate and the hybrid or breed of chickens from a flock with a stocking density above 33 kilograms per m2 of usable area are treated as relevant food safety information and included in the FCI. See points 2.1.3 and 2.1.4 below.
References:
- Council Directive 2007/43 (EC)
- SI No 3033/2010 The Welfare of Farmed Animals (England) (Amendment) Regulations 2010
- SI No 2713/2010 (W229) The Welfare of Farmed Animals (Wales) (Amendment) Regulations 2010
1.4 FSA Operational staff role
Food Standards Agency Operational staff role
| Inspection and verification | By | Frequency | Time code |
|---|---|---|---|
| Review FCI and use information for ante-mortem inspection | OV / MHI if AM on farm of any species | One per batch from a producer or for individual animals | INSP |
| Carrying out ante-mortem inspection and recording data | OV | Individual animals Batches of poultry Recording by animal or batch | INSP |
| Carrying out post-mortem inspection and recording data | OV or MHI OV for meat with abnormalities. | Individual carcases and offal. Recording by carcase or batch | INSP |
1.4.1 Implementation of CCIR
The OV shall record and evaluate the results of official controls. IT tools have been developed allowing the collection and communication of the inspection results to abattoir FBOs and producers. The IRIS system is now available for all species.
FSA staff can access IRIS support pages, along with Inspection Results Templates and Condition Reference cards from IRIS2 guidance in internal files.
Access IRIS to input information.
Reference: (EU) 2019/627, Article 39.
2. Food Chain Information
In this section
2.1 FCI: Poultry
2.1.1 Background
Since 01 January 2006, it has been a requirement that FCI is supplied in respect of poultry intended for human consumption.
The minimum information to be provided by the FBO rearing animals (farmer or producer), not less than 24 hours before the arrival of the poultry at the slaughterhouse, is contained in the form ‘Poultry FCI’ in annex 11. This form has been provided by the FSA to all slaughterhouse FBOs.
Reference: (EC) 853/2004, Annex II, Section III, 3 (a) - (h).
2.1.2 Categories of chickens
For the purposes of entry of the FCI details into IRIS, one of three categories should be used for chickens:
Categories of chickens
| Category | Description |
|---|---|
| Broilers | All chickens reared specifically for food production (as meat). This includes poussin, slow-growing organic birds and cockerels specifically reared for meat |
| Hens | Reared for the production of eggs for food consumption |
| Poultry | Cockerels and hens used for breeding and not the prime purpose of food production, or rare cases of other poultry that do not classify as ‘broilers’ or ‘hens’ |
2.1.3 Council Directive 2007/43/EC
EU Council Directive (EC) 2007/43 (The Broiler Directive) lays down minimum rules for the protection of conventionally reared meat chickens (broilers) on holdings with 500 or more birds.
Under this Directive, the maximum on-farm stocking density (SD) for conventionally reared meat chickens is 33 kg/m².
SD above 33 kg/m² and up to 39 kg/m² is allowed, providing that the keeper complies with the extra requirements as detailed in the legislation listed below.
References:
- SI No 3033/2010 The Welfare of Farmed Animals (England) (Amendment) Regulations 2010
- SI No 2713/2010 (W229) The Welfare of Farmed Animals (Wales) (Amendment) Regulations 2010
2.1.4 Additional poultry FCI requirements under Council Directive 2007/43/EC
In relation to FCI, several pieces of data are considered relevant food safety information for flocks above 33 kg/m². These are:
- the cumulative daily mortality rate (CDMR) for each house
- information on the hybrid or breed of chicken for each house
Note: See annex 1 for an example of a completed CDMR table.
2.1.5 Poultry slaughterhouse - FBO responsibility
The FBOs of establishments processing poultry must request, receive, check and act on FCI. They must not accept poultry for slaughter unless they have requested, received and acted upon the information.
Receipt should normally be no less than 24 hours before delivery of the birds.
The FBO must make the FCI, including details of the numbers of dead on arrival, available to the OV. The FBO must notify the OV of health concerns before the OV carries out an ante-mortem inspection.
Reference: (EC) 853/2004, Annex II, Section III Points 1, 2 and 5.
2.1.6 OV responsibility
The OV must check the FCI provided for completeness and contents as a part of the ante-mortem inspection.
The OV is entitled to request any additional data from the producer. For example, when presented with a very high CDMR and no explanation is on the FCI for this, it is reasonable to request the complete set of daily mortality rates (for that particular flock’s production cycle) to fully understand at what stage of the production cycle significant mortality occurred. This should help the OV evaluate the health and welfare status of the birds on arrival at the slaughterhouse and determine whether there are immediate concerns regarding the health and welfare of any remaining birds at the site.
FCI should also be taken into consideration when post-mortem inspection is carried out.
The hierarchy of enforcement should be followed if any of the required FCI elements are missing, or the information is misleading (see point 4.2 below).
Legislation establishes that the OV must impose conditions under which animals must be dealt with under a specific scheme for the eradication or control of a specific disease, such as brucellosis or tuberculosis, or zoonotic agents such as salmonella, under direct supervision. The Competent Authority (CA) must also determine the conditions under which such animals may be slaughtered. These conditions are designed to minimise the contamination of other animals and the meat of other animals.
Reference: Regulation (EU) 2019/627, Chapter III, Article 43 Point 6.
2.1.7 FCI: Salmonella on-farm testing (National Control Programme)
There is a statutory requirement for salmonella on-farm testing of most chicken and turkey flocks under the requirements of the UK Salmonella National Control Programmes (NCPs). Producers are required to take boot-swab samples (or other sample types permitted under the NCP) from the poultry bedding or the environment at the farm. The sectors covered, and the producers to which the statutory NCP requirements are applicable, are detailed in the tables below.
All birds (unless exemptions explained in the tables apply) must arrive at the slaughterhouse with the salmonella NCP test(s) result(s) and the date(s) of the sampling of their specific flock recorded in the FCI. UPDATED: [The FCI for the batch must include at least:
- the most recent NCP salmonella test result for the specific flock(s)
- the date(s) the sample(s) was/were taken
- any significant flock disease
- any treatment/medication prescribed
Salmonella Enteritidis and Salmonella Typhimurium have accounted for the majority of cases of human salmonellosis and have consistently been the most commonly implicated pathogens in general outbreaks of food-borne disease. These are referred to as ‘salmonella-regulated serovars’.
If an operator or an official sample test positive for Salmonella Enteritidis or Salmonella Typhimurium (including monophasic Salmonella Typhimurium) the flock will be considered high-risk positive, and the operator must declare it on the FCI and agree to its acceptance in advance with the FBO at the slaughterhouse.
The Animal and Plant Health Agency (APHA) will also take random NCP official samples each calendar year from a single flock on 10% of holdings with more than 5,000 broilers or more than 500 fattening turkeys or in cases where there is no evidence of the required level of NCP testing or NCP rules have not been followed in a specific flock or flocks.
Note: If the date of on-farm sampling is more than the number of weeks permitted in the tables below, the birds can still be slaughtered. This should be according to the specific measures set in point 2.1.12 ‘OV action where the salmonella result has not been recorded on the FCI or sampling is outside the sampling window’.
UPDATED: [Broilers: salmonella sampling requirements
| Type | Sampling requirements | Applicable to | Exclusions |
|---|---|---|---|
| Broilers 81 days old or less (footnote 1) | Within 3 weeks before slaughter | Keepers of 2,000 or more broiler chickens at any time in 12 months |
Keepers that produce birds for private domestic use (the birds do not enter the market). OR Keepers that supply the consumer direct (such as through farm-gate sales or local retailers). |
|
Broilers 82 days old or more AND Broilers that have organic certification from an UK approved organic control body |
Within 6 weeks before slaughter | Keepers of 2,000 or more broiler chickens at any time in 12 months |
Keepers that produce birds for private domestic use (the birds do not enter the market). OR Keepers that supply the consumer direct (such as through farm-gate sales or local retailers).] |
Chicken breeders: salmonella sampling requirements
| Type | Sampling requirements | Applicable to | Exclusions |
|---|---|---|---|
| Adult breeding chickens (gallus gallus) |
Once birds are moved into the breeding unit, sampling must happen every 2 weeks. Chicken breeder flocks may sample every 3 weeks if both:
|
Keepers of 250 or more breeding chickens at any time within a 12-month period | Flocks that only produce eggs or chicks for scientific or research purposes (that are not for human consumption) |
Laying hens and flocks: salmonella sampling requirements
| Type | Sampling requirements | Applicable to | Exclusions |
|---|---|---|---|
| Laying chickens producing eggs for human consumption |
Pullets (also known as young/rearing hens) must be sampled within two weeks before moving to the laying unit. Adult egg-laying flocks must start the sampling between 22 and 26 weeks old and at least every 15 weeks during the laying production. |
All commercial laying chicken flocks that produce table eggs (Class A eggs) for human consumption. |
|
UPDATED: [Turkeys: salmonella sampling requirements]
| Type | Sampling requirements | Applicable to | Exclusions |
|---|---|---|---|
| Fattening turkeys (birds reared to produce meat for human consumption) |
Turkeys slaughtered at 100 days old or less: Sampling within 3 weeks before slaughter. Turkeys slaughtered at more than 100 days old: Sampling within 6 weeks before slaughter. Turkeys that have organic certification from an UK approved organic control body: Sampling within 6 weeks before slaughter. |
Keepers of 500 or more fattening turkeys at any time in 12 months. |
Where APHA has granted a keeper an exemption from NCP sampling (applicable in GB only), the exemption must be recorded on the FCI. The aforementioned exemption applies only to farms rearing between 500 and 10.000 turkeys within any 12-month period, where all meat is supplied either directly to the final consumer or to local retail establishments, and only during the two weeks immediately preceding Christmas and Easter. (footnote 4) |
| Turkey breeders (footnote 5) |
Turkeys slaughtered at 100 days old or less: Sampling within 3 weeks before slaughter. Turkeys slaughtered at more than 100 days old: Sampling within 6 weeks before slaughter. Turkeys that have organic certification from an UK approved organic control body: Sampling within 6 weeks before slaughter. |
Keepers of 250 or more breeding turkeys over the course of a 12-month period. | Flock that produces eggs or poults (young turkeys) for scientific or research purposes only (that are not for human consumption).] |
‘Local/Locally’ in the exemption columns of the above tables refers to producers selling directly to consumers at farmers’ markets or retailers in any of the following:
- the county where the holding is
- the counties next to the holding’s county
- anywhere up to 30 miles (or 50 Km) from the borders of the holding’s county
Nevertheless, the rules on only selling locally are lifted in the fortnights leading up to Easter and Christmas for fattening turkeys.
The requirement for statutory salmonella sampling at the farm does not apply to other poultry species (for example ducks, quails). However, whilst there is no testing requirement, salmonella status may be required to be included in the FCI under voluntary assurance or good practice schemes. The FCI must state:
- the date(s) on which the salmonella NCP sample was taken
- whether the result(s) was/were positive or negative
- if positive, detail of the serotype or at least the serogroup (A,B,C,D,E) result
In such cases, it is expected that the FBO has procedures in place to deal with this hazard by establishing procedures based on HACCP principles to minimise the risk of potential cross-contamination at all stages when handling positive batches.
2.1.8 On farm restrictions: OV actions
In some circumstances, the NCP test results can lead to a flock being placed under restriction when positive for Salmonella enteritidis, Salmonella typhimurium or monophasic strains of Salmonella typhimurium (antigenic formula salmonella 1,4,[5],12:i-).
In these cases, the birds can only move under an APHA movement licence. The OV can expect to receive the APHA movement licence either at the time the FCI documents are received or on arrival of the birds at the slaughterhouse. The number of birds in the batch should be cross checked with the details on the movement licence (which may cover more than one consignment of birds) and any further batches expected at the slaughterhouse. If any anomalies are detected, the APHA office that issued the movement licence should be contacted. Such licences may have been issued by either the local APHA office or by Business Support (SSC), Worcester.
However, a restriction notice is not always served on a salmonella positive flock. If no restriction notice has been served, no movement licence will have been issued by APHA, even if the FCI states that the birds have tested positive for salmonella. Whether or not a restriction notice is issued to a particular farmer will depend on the situation and the specific sector NCP. If no movement licence is received with a high-risk positive flock, the OV should contact the APHA office to confirm if the farm is under restriction.
References:
- REUL No 2160/2003
- REUL No 200/2010 (implementing legislation for breeding chickens)
- REUL No 517/2011 (implementing legislation for laying chickens)
- REUL No 200/2012 (implementing legislation for broilers)
- REUL No 1190/2012
- SI No 2007/3574 The Control of Salmonella in Poultry (England) Order 2007
- SI No 2008/524(W50) The Control of Salmonella in Poultry Scheme (Wales) Order 2008
- SI No 2008/263 the Control of Salmonella in Poultry Scheme Order (Northern Ireland) 2008
- SI No 2009/229 The Control of Salmonella in Poultry (Breeding, Laying and Broiler Flocks) (Scotland) Order 2009
- SI No 2009/260 The Control of Salmonella in Broiler Flocks (England) Order 2009
- SI No 2009/441(W46) The Control of Salmonella in Broiler Flocks (Wales) Order 2009
- SI No 2009/205 The Control of Salmonella in Broiler Flocks Scheme Order (Northern Ireland) 2009
- SI No 2009/3271 The Control of Salmonella in Turkey Flocks (England) Order 2009
- SI No 2010/65(W15) The Control of Salmonella in Turkey Flocks (Wales) Order 2010
- SI No 2010/248 The Control of Salmonella in Turkey Flocks Scheme Order (Northern Ireland) 2010
- SI No 2009/417 the Control of Salmonella in Turkey Flocks (Scotland) Order 2009
The primary framework legislation, REUL 2003/99/ and 2160/2003, implementing legislation for NCPs specifically deals with salmonella control at all relevant stages of the food chain, but principally at the farm.
2.1.9 FBO action where a positive test result for a regulated salmonella serovar (high risk) is recorded in the FCI
Where a positive test result indicates the presence, or the suspicion of the presence, of a regulated salmonella serovar in the FCI, these flocks must be treated as high risk to public health. This applies to the following:
- Salmonella enteritidis
- Salmonella typhimurium
- monophasic Salmonella typhimurium (1,4,[5],12:i-)
- group D salmonella (suspect enteritidis)
- group B (suspect typhimurium / monophasic typhimurium)
FBO actions when accepting a high-risk salmonella flock:
- alert the OV to the FCI content regarding salmonella and inform the OV of the procedures in place to process the flock
- organise the slaughter plan for the day so that the affected batch(es) are slaughtered at the end of the production day to minimise the risk of cross-contamination
- after slaughter of the affected batch(es), undertake a full cleansing and disinfection of all equipment and machinery, including changing the water in the scalding tank(s), and renewing the water in the spin chiller(s)
- where a high-risk salmonella positive batch has been slaughtered during the production day (either in error or on welfare grounds), then the production should be stopped as soon as the affected batch has been slaughtered, and full cleansing and disinfection as above must take place before any further slaughtering commences
- measures should be taken to minimise the risk of potential cross-contamination at all stages when handling high risk salmonella positive batches
- the FBO must follow their own documented procedures, based on the HACCP principles, as regards to placing the meat on the market
FBO actions regarding decisions concerning meat from a high-risk salmonella flock:
The carcases from high risk salmonella positive batches cannot be released for human consumption unless they meet the requirements of the table below.
The 3 options below are available at the slaughterhouse if the FBO accepts processing the high-risk salmonella flock(s).
Notes: Until test results are received, the meat will have to be retained in the slaughterhouse (if necessary frozen) adequately identified and stored separately from other meats.
Note: For the purpose of these instructions, a flock is defined as a group of birds reared in the same house within the same farm. For birds not confined solely to a house, a flock is equivalent to a group of birds that physically share a designated area.
Note: for further details on how to take salmonella samples at the slaughterhouse refer to Chapter 4.3 (Verification of microbiological criteria).
1. Undertaking a Sentinel test
The FBO has the option of accepting high-risk salmonella positive flocks and carrying out a sentinel test of the affected flock, by sampling 15 neck flaps from a batch of 150 birds at the abattoir under Regulation (EC) 2073/2005, point 1.28 of Annex I, Chapter 1 –absence in 5 samples of 25 gr each (neck flap)–.
If the result of this test is negative, the flock should still be processed as a high-risk salmonella positive as a preventive measure to ensure the protection of public health and to minimise any potential cross-contamination of the slaughterhouse facilities, but the meat can be released for human consumption as fresh meat.
The remaining birds from this flock have to be processed in the same abattoir, and immediately after the results are obtained. Thinning would not be an option in these circumstances. The 150-batch sample is considered to be representative of assessing the risk status of the fresh meat to be placed on the market.
2. Undertaking poultry carcases (neck flap) or poultry portions sampling
Accept the flock and test the carcases for salmonella spp. (neck flap) to ensure they comply with the Process Hygiene Criteria under point 2.1.5 of Annex I, Chapter 2 of Regulation (EC) 2073/2005.
If salmonella is isolated, serotype the samples for S. enteritidis and S. typhimurium to ensure compliance with the Food Safety Criteria under point 1.28 of Annex I, Chapter 1 (absence in 5 samples of 25 gr each).
For the salmonella analysis for fresh poultry meat other than poultry carcases (for example, from portions or when the neck flap has been removed) 5 samples of at least 25 g of the same batch shall be collected ensuring that the sample contains skin and a thin surface muscle slice to form each sample unit.
3. Processing the meat by heat treatment or other treatment capable of eliminating the hazard at an establishment other than retail
If the meat is not tested or if positive for a salmonella regulated serovar after testing as described in points 1 and 2 above, the meat can be processed by a treatment eliminating the hazard (for example industrial heat treatment).
This treatment may only be carried out by FBOs other than those at the retail level. Untreated meat that has tested positive will have to be discarded as category 2 animal by-product.
FBO Actions at the slaughterhouse for NCP high-risk salmonella positive flocks
| Salmonella enteritidis or typhimurium fresh meat test result (sampled at the slaughterhouse) | FBO action | Meat and offal | Animal by products (ABP) |
|---|---|---|---|
| Negative (-) | None | Fit for human consumption as fresh meat in accordance with the food hygiene regime | Category 3 in accordance with the normal ABP regime |
| Positive (+) |
Processing by a treatment eliminating the hazard in question (for example, industrial heat treatment or another treatment that eliminates salmonella). This treatment may only be carried out by food business operators other than those at retail level. |
Fit for human consumption as meat product in accordance with the food hygiene regime | Category 3 in accordance with the normal ABP regime |
| Positive (+) | Not treated (because of a commercial decision) | Unfit for human consumption | Category 2 |
| Not tested |
Processing by a treatment eliminating the hazard in question (for example, industrial heat treatment or another treatment that eliminates salmonella). This treatment may only be carried out by food business operators other than those at retail level. |
Withdrawal of products that are not at retail level for either further treatment or disposal |
Category 3 in accordance with the normal ABP regime |
| Not tested | Not treated (because of a commercial decision) | Unfit for human consumption | Category 2 |
| Not tested | Already placed in the market or ready to be placed in the market (for example, incorrectly completed FCI at the time of slaughter) | Withdrawal of products that are not at retail level for either further treatment or disposal |
If ABPs still traceable:
|
| Not tested –culled at the abattoir – not intended for human consumption | Culling in a slaughterhouse should be permitted only in exceptional circumstances and after being permitted by the CA. Further information provided in subtopic 2.1.15 | Unfit for human or animal consumption | Category 2 |
2.1.10 FBO action where a positive result for lower risk salmonella serovar is recorded in the FCI
Where a positive test result for a lower risk salmonella serotype (other than Salmonella enteritidis or Salmonella typhimurium as detailed in point 2.1.9 above) is indicated on the FCI, the FBO should take the following actions:
- alert the OV to the FCI content regarding salmonella and inform the OV of the procedures in place to process the flock
- organise the slaughter plan for the day so that the affected batch(es) are slaughtered at the end of the production day, or if this is not possible on welfare grounds, at the end of a production run or just before an operational break
- where a positive batch has been processed in the middle of a production run, as soon as the affected batch has been processed, a thorough wash down (full cleansing and disinfection as detailed above for high risk is not necessary) of the plucking and evisceration room (including equipment) must be undertaken before any further processing re-commences. This is to minimise the risk of cross contamination for the following batches
- in any case, after the finish of production for the day, a full cleansing and disinfection of all equipment and machinery, including changing the water in the scalding tanks, and renewing the water in the spin chillers must be undertaken
- following production, in the absence of any relevant AM or PM findings, the carcases can enter the food chain as normal
Note: Poultry meat preparations, poultry minced meat and meat products tested under Regulation (EC) 2073/2005 must be negative for all salmonella serotypes, not just S. typhimurium or S. enteritidis. For more information please refer to MOC Chapter 4.3 Verification of microbiological criteria.
Note: Legislation requires that FBOs check FCI and act upon the information received. In the case of salmonella positives, the FBO should have the procedure to follow included in their HACCP-based food safety management system.
2.1.11 OV action where a positive salmonella test result is recorded in the FCI
The OV is to:
- check which salmonella serotype is detailed on the FCI (or if serotyping is still pending, assume serogroups B and D are high-risk flocks unless Salmonella enteritidis or Salmonella typhimurium have already been excluded)
- check the date of the sampling and confirm compliance with the period required as per the table above in point 2.1.7
- check that the high / low-risk procedure has been followed following the FBO’s HACCP-based food safety management system
- notify the inspection team that the flock is positive, and ensure that the appropriate judgement on pericarditis is followed in accordance with the information contained on the Pericarditis Poultry Condition card (see chapter 2.4 on ‘Post-mortem, health and identification marking’, section 7)
- in case of specific incidents or 'force majeure' such as lengthy breakdowns or road accidents (as an example) that might have a considerable impact on the welfare of the animals, the situation might need to be dealt with on a case by case basis. If this occurs, the OV must contact the poultry portfolio representative for further assessment of the situation and guidance on how to proceed with the salmonella positive flock(s)
- ensure that the relevant cleansing and disinfection procedure is followed (as detailed in the previous sub-topics) after processing salmonella positive flocks
Where non-compliance is found, action should be taken in accordance with the hierarchy of enforcement as outlined in Chapter 7 on ‘Enforcement’.
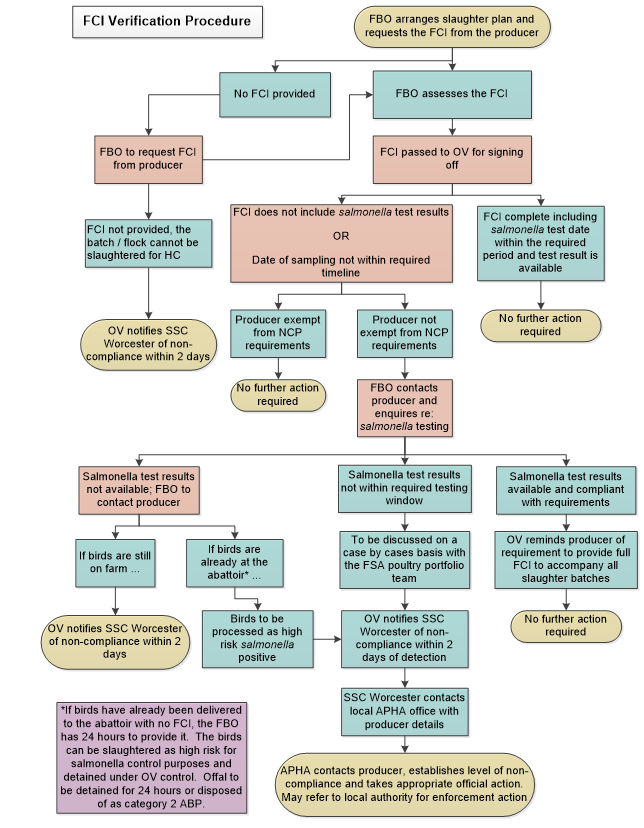
2.1.12 OV action where the salmonella result has not been recorded on FCI, or the NCP salmonella sampling is outside the sampling window.
In the first instance, the OV should request that the FBO contact the primary producer of the batch to determine whether an oversight has occurred and request the appropriate information is made available.
Where the flock
- was not eligible to be tested under the requirements of the NCP, the batch can be slaughtered as per normal procedures
- was eligible for testing, and the primary producer confirms that the test result is available, the OV must ensure that a copy of the test result is sent to the slaughterhouse. Once received by the FBO, action should be taken with the consignment in accordance with the test result received
Where this fails to resolve the issue, and no test results are available, the batch must be considered to be of unknown salmonella status.
If the flock is still at the farm, then the OV is to contact APHA within 2 working days to discuss the case
If the birds are already in the abattoir, these should be processed as if a high-risk salmonella result had been received. The OV is to contact APHA within 2 working days for information purposes purposes and include the FSA poultry portfolio in the notification, or notify the portfolio if a phone call was made.
FSA poultry portfolio contact details:
APHA contact details:
- CSCOneHealthSalmonella@apha.gov.uk
- telephone - 0345 601 4858
Details for APHA should include contact details of the affected farm and specific flock(s) as per the FCI.
When the examination of the sample does not start within 48 hours following the time of receipt of the samples by the laboratory and within four days from the date of sampling, as per Regulation 200/2012 requirements, the sample is rendered as not valid for preslaughter NCP purposes, and the flock must be processed as a high-risk salmonella flock as described in section 2.1.9 of this chapter. These cases must be reported to APHA.
Alternatively, the flock can be sampled/tested again if the birds are still on the farm or have been returned to the farm following the identification of this issue.
Finally, where the most recent test was taken earlier than permitted under NCP rules and outside the sampling window, the case is to be discussed individually with the FSA poultry portfolio team (poultry.portfolio@food.gov.uk).
A decision will be made based on flock status, past history, epidemiological assessment and length of time outside the window.
2.1.13 Salmonella group rather than serotype provided
In instances where the salmonella group is provided instead of the serotype, the batches can still be processed as follows:
Result
- salmonella groups D or B*
- salmonella groups C, G or E
Action:
- as high-risk salmonella positive
- as low-risk salmonella positive
* The current serotyping process, for Salmonella typhimurium and monophasic strains especially, can be lengthy. The test process can, at an earlier stage, rule out the serotype being Salmonella typhimurium. It has therefore been agreed that an official or a NCP approved laboratory report, confirming that the flock is salmonella positive, serogroup B, but that the isolate is not Salmonella typhimurium (based on initial antigen determination) is acceptable for the flock to be processed as low-risk salmonella positive.
2.1.14 Additional information to consider regarding salmonella positive results
Once a salmonella positive result is obtained in a flock, the salmonella status does not usually change, even if subsequently collected NCP sample test results for that flock are negative:
- the exception is if a subsequent officially collected confirmatory sample negates this result (official confirmatory samples are only collected by APHA (GB) or DAERA (NI) and are not collected in every positive breeding or laying flock)
- flocks are to be processed as salmonella positive high/low risk if there has ever been a positive salmonella result unless a subsequent officially collected confirmatory sample was negative (in which case the original NCP sample result is officially deemed a false positive)
Long term rearing birds (for example, fattening turkeys, slow reared broilers or breeding flocks) can recover to negative after an initial salmonella positive result:
- in these cases, the statutory salmonella testing required before slaughter should confirm the latest negative test of the flock
- the FCI must however show all salmonella testing results and the birds will still be considered positive for a regulated serovar under the NCP. The FSA internal procedure for these cases is that, if there are no other concerns, each case must be discussed individually with the Poultry Portfolio team (poultry.portfolio@food.gov.uk) that will confirm if the flock should be treated as positive or as negative
- if the portfolio confirms that the latest negative result can be considered, in the case of a previous positive for high-risk salmonella serovars, the flock can be slaughtered as if it was a low-risk salmonella serovar. In the case of a previous positive for low-risk salmonella serovars, the flock can be slaughtered as any normal flock
If the salmonella positive result is linked to a serovar used for vaccinating the flock (which should be stated in the laboratory result), this flock is not considered as salmonella positive for the purposes of the birds being slaughtered for human consumption, and the flock can be processed as any other normal flock.
2.1.5 Culling of a flock positive to salmonella regulated serovar in the slaughterhouse
Culling flocks positive to a salmonella regulated serovar in a slaughterhouse is permitted only in exceptional circumstances, if authorised by the competent authority (FSA), and when all alternative options have been exhausted. Permission will be granted on a case-by-case basis.
An example of exemptional circumstances could be welfare grounds in cases where culling companies are not available to cull the birds on the farm in time to prevent welfare issues due to increased stocking density.
When the results of an NCP test are positive for a regulated salmonella serovar in a flock of birds, APHA will inform the FSA poultry portfolio. If the bird producer cannot cull the birds at the farm, they will contact the slaughterhouse directly, asking the FBO if they would accept the birds either for processing, following one of the options described in point 2.1.9, or for being culled at their site and disposed of as Category 2 animal by-products.
If the FBO is willing to accept the birds, the decision to grant permission for the culling will be undertaken by the local FSA team (FVL and FVC) in consultation with the FSA poultry portfolio (poultry.portfolio@food.gov.uk).
The decision will be based on the establishment demonstrating that they have specific procedures to process the birds hygienically and minimise the risk of spreading the disease. These procedures must cover at least the following:
- total segregation of the positive flock from other live birds
- the disposal of the culled birds, blood and feathers as Category 2 animal-by-products
- after culling the affected batch(es), the FBO undertakes a full cleansing and disinfection of all equipment and machinery, including the scalding tank(s), and the spin chiller(s) once emptied
- a thorough cleansing and disinfection of crates and modules used to transport live birds, vehicles and all areas used to process the birds, for example bleeding area, plucking equipment, etc
- evisceration is not recommended, but if the particularities of the line do not allow the eviscerations to stop, these areas must be subsequently cleansed and disinfected before processing birds intended for human consumption
- verification that the cleansing and disinfection have been satisfactory
The OV shall supervise the culling process and verify that the cleaning and disinfecting procedures have been effective.
Legal reference: Annex III, Section II, Chapter IV, point 10 of Regulation (EC) 853/2004 allows the use of white meat slaughterhouses, when permitted by the competent authority, for slaughtering sick or suspect animals and when applying disease eradication or control programmes. Please note that this provision is not available for red meat slaughterhouses.
2.2 FCI: Pigs
2.2.1 Background
FCI for pigs was fully implemented from 1 January 2008.
2.2.2 Pigs slaughterhouse - FBO responsibility
FBOs must not accept pigs for slaughter unless they have requested, received and acted upon the FCI.
After deciding to accept the pigs for slaughter, the FBO must make the FCI available to the OV without delay. The FBO must notify the OV of health concerns before the OV carries out an ante-mortem inspection.
Reference: (EC) 853/2004, Annex II, Section III, Points 1, 2 and 5
It is the responsibility of slaughterhouse FBOs to decide on the FCI that they require and to request this FCI from the FBO rearing the animals (farmer or producer). Guidance on the minimum requirements for FCI can be found on the FSA website.
Reference: (EC) 853/2004, Annex II, Section III, Point 3 (a) - (h).
2.2.3 Methods of receiving pig FCI
Since 1 April 2012, pig keepers in England and Wales are required to report movements (including pigs from Scotland) using the electronic AML2 online system (eAML2) operated by the British Pig Executive (BPEX). Refer to Chapter 2.5, Section 2.11 for further details on pigs’ identification requirements.
To be legally compliant, pig movements must be reported through the eAML2 online system or by contacting the eAML2 free-to-use bureau service by phone (to get a printed copy for the movement).
eAML2 contact details:
- Email: eaml2@ahdb.org.uk
- Telephone: 0844 335 8400
- Monday to Friday, 9am to 5pm
The FBO should receive the FCI by at least one of the following routes:
- via eAML2 online system
- included in the haulier summary (HS), a document required by Trading Standards to accompany every load in transit, which contains the movement and FCI details
- on the ‘old style’ FCI paper form
Note: In Scotland, with effect since 1 December 2011, details of pigs moving to slaughter should be notified to the ScotEID movement reporting database electronically, by telephone or in writing. This includes movements of pigs from Scotland to England and Wales for slaughter.
Reference: Regulation (EC) 853/2004, Annex II, Section III, Point 3 (a) - (h).
2.2.4 Pigs arriving without FCI
FCI must be provided for all animals slaughtered for human consumption, within 24 hours of their arrival.
The OV may permit animals without FCI to be slaughtered, but the health mark must be withheld until the FCI has been provided and examined.
Pending final judgement, the carcases and offal must be stored separately from other meat (subject to the provision below).
When animals arrive without FCI but are slaughtered and the meat held pending the arrival of the FCI, the meat shall be declared unfit for human consumption and disposed of as an animal by-product if no FCI is provided within the 24-hour period, as required by the Regulations.
Note: See section 4 below on ‘Verification and Enforcement’ for further information.
2.2.5 Housing system/Controlled housing conditions
Pig producers must declare the housing system used for their pigs on the FCI.
‘Controlled housing conditions’ include a range of measures that reduce the risk of the pigs being infected with trichinella and therefore these pigs are exempt from trichinella testing at the slaughterhouse. Importantly, the definition does not exclude pigs that have outdoor access, provided that the outdoor access does not present a risk of introducing trichinella into the holding.
Republic of Ireland (RoI) has, to date, not put in place a mechanism whereby housing can be deemed to meet the conditions specified in Article 1 and Annex IV of retained Regulation (EU) No 2075/2005. Therefore, all pigs born and reared in RoI, which are slaughtered in slaughterhouses in England or Wales, shall be tested for trichinella, regardless of the housing system recorded on the FCI.
Refer to Chapter 2.4, Section 5 for detailed information on trichinella testing.
Reference: Regulation (EU) 2015/1375, Annex IV, Chapter I.
2.3 FCI: Horses
2.3.1 Background
FCI for horses was fully implemented from 1 January 2009.
It is the responsibility of slaughterhouse FBOs to request this FCI from the FBO rearing the animals (producer, owner or keeper).
With effect from 23 February 2015, FCI, in addition to the passport for individual equines, must accompany all equines consigned for slaughter for human consumption. Note that FCI is also required for horses originated from wild and semi-wild horses living in designated areas to which certain identification derogations apply.
Requesting and receiving FCI is the responsibility of the slaughterhouse operator.
Note: the rules for horses apply to all equidae (donkeys, asinine, mules). Instructions in this chapter refer to horses for purposes of simplification.
Reference: (EC) 853/2004, Annex II, Section III, 3 (a) - (h).
2.3.2 Horse slaughterhouse FBO responsibility
FBOs of establishments processing horses must request, receive, check and act on FCI. They must not accept horses for slaughter unless they have requested, received and acted upon the information.
A revised model FCI document is attached in annex 3.
After deciding to accept the horses for slaughter, and after conducting identity checks, the FBO must make the passport and FCI available to the OV without delay. The FBO must notify the OV of health concerns before the OV carries out AMI.
Reference: (EC) 853/2004, Annex II, Section III, 1, 2, 5.8.
Note: See chapter 2.5 on ‘Animal identification’, section 4.10 on ‘Verifying eligibility of horses’ for detailed guidance.
2.4 FCI: Other species
2.4.1 FCI implementation for other species (other than poultry, pigs, horses and veal calves)
For species other than poultry, pigs, horses and veal calves, FCI was implemented from 1 January 2010.
As with other species, the FSA has provided guidance on the ‘minimum elements’ of FCI required and model documents have been developed. This has been made available on the FSA website. FBOs may however choose to request additional information.
For farmed game, information should be provided in the FBO’s declaration made at the time of slaughter which, if correctly completed, contains all the elements required for FCI.
There is no requirement for the provision of FCI for wild game animals; this is replaced by the hunter’s declaration.
2.4.2 FCI in cases of on farm emergency slaughter
The Official Health Certificate (GBHC056) that accompanies the bodies of animals subjected to emergency slaughter outside the slaughterhouse, does not contain any of the elements required for FCI, and therefore Food Chain Information must also accompany the body of the animal. This should be completed and signed by the owner of the animal.
2.4.3 Additional FCI requirement for cattle
There is a requirement within the ‘minimum elements’ of FCI for cattle that a declaration is made by the keeper, specifying the bovine tuberculosis (TB) status of the holding.
This will assist in identifying cattle that have tested negative but come from restricted herds, or young animals aged less than 8 weeks, which arrive under a general licence and are indistinguishable from animals arriving from non-restricted herds.
In such cases, the FCI will determine the origin of the animals, and there is a requirement for the OV to be in attendance during slaughter.
2.4.4 Additional FCI requirement for other species susceptible to bovine TB
Food chain information, incorporating a declaration regarding the TB status of the holding, must be provided for farmed animals such as camelids, bison, water buffalo and deer that are slaughtered on farm. The FCI must accompany the carcases to the slaughterhouse.
2.5 FCI receipt and check
2.5.1 FCI receipt by the OV
The OV should receive the FCI report from the slaughterhouse FBO at least 24 hours in advance of arrival of the animals. However, FCI can be received at the same time as the animals providing that:
- it does not jeopardise the objectives of (EC) 853/2004
- it does not cause serious disruption in the slaughterhouse activity
Where animals have undergone ante-mortem inspection by an OV at the holding of provenance and have the relevant certificate, the FCI may accompany the animals rather than arriving 24 hours in advance.
Reference: (EU) 2019/624, Article 5, Paragraph 2(f).
Poultry note
FSA considers that, because of the organisation of the poultry industry and the need to use FCI to plan the slaughter of flocks, it is necessary that FCI is received in advance of the arrival of the poultry at the slaughterhouse.
FSA is encouraging slaughterhouse FBOs to treat the 24 hours period as a minimum period and to request FCI further in advance if this is necessary to make appropriate arrangements for specific flocks (for example, to plan the slaughter of a flock which has tested positive for salmonella at the end of a shift / day).
Other species
The FSA has elected to allow FCI to be sent to the slaughterhouse operator with the animals (FCI is not required to be sent 24 hours in advance). However, if there is any information on the FCI which might result in serious disruption to the slaughterhouse activity, the FCI must be received in good time before the animals arrive. In addition, FBOs are recommended to obtain FCI long enough in advance of delivery to the slaughterhouse to enable them and the OV to take any necessary action.
Additional declaration
An animal may be accompanied by an additional farmer’s declaration describing that the animal is known or suspected to be injured or showing signs of abnormality. The OV shall verify that the declaration reflets the status of the animal.
Reference: See annex 18 of this chapter (Additional Food Chain Information) for an example of a model document to accompany animals showing signs of a disease or condition that may affect the safety of meat derived from them.
2.5.2 OV role
The OV is to check and analyse relevant information from the FCI report and may take any of the following decisions:
- animals with a disease or condition that may be transmitted to animals or humans through the handling or eating of meat must be rejected for slaughter and killed separately under conditions such that other animals cannot be contaminated and declared unfit for human consumption
- change slaughterhouse process (for example, reduce line speed or increase number of inspectors)
- slaughter animals / batch of animals last (for example, if known to carry a pathogenic organism)
- detain animal(s) or carcase(s) for further testing
2.5.3 Animals with no FCI
If animals arrive at the slaughterhouse without FCI, the FBO must notify the OV. The OV should agree the procedure with the FBO in advance.
Reference: (EC) 853/2004, Annex II, Section III, 6.
The OV may permit the slaughter of animals if the FCI is not available. In such cases the OV must detain carcases of animals slaughtered in the absence of FCI, and their related offal, pending receipt of FCI.
Reference: (EU) 2019/627 Article 40.
Before permitting the slaughter of animals without FCI, the OV must ensure that:
- there are adequate facilities for the separate storage of carcases and their offal
- arrangements are in place to identify these in the slaughter line so that they are not inadvertently health marked
If the OV decides to permit slaughter they will need to confirm this in writing to the FBO.
The Regulations provide that, if FCI is not received within 24 hours of the animal’s arrival at the slaughterhouse, all meat from the animal is to be declared unfit for human consumption.
FCI must be provided, within 24 hours of their arrival, for all animals slaughtered for human consumption.
When the OV does not permit the slaughter of animals (for example, where there are no facilities to store carcases separately), the animals may, subject to animal health and welfare considerations, be kept in the lairage until the food chain information is provided. If this information cannot be supplied or the FBO does not wish to keep animals in the lairage then the animal(s) must be killed separately from other animals and the meat declared unfit.
Reference: (EC) 2019/627 Article 40. Further information is available in section 4 on ‘Verification and enforcement’ in this chapter.
2.5.4 The Cascade principle in the use of medicines
The Cascade principle allows veterinary surgeons to legally prescribe medicines that are not authorised, at a different concentration or for another specie for a relevant clinical case. The Cascade is a risk-based decision tree to help veterinary surgeons decide which product to use when and at which concentration when no authorised veterinary medicine is available or authorised.
A veterinary surgeon prescribing or administering a medicine to food-producing animals under the Cascade principle must specify an appropriate withdrawal period. When setting this withdrawal period, the veterinary surgeon must consider known information about the use of the product on the authorised species and concentrations when prescribing under the Cascade principle.
Where the product is not used as authorised, for example, when a higher dose or longer duration of treatment is used, or a species for which the product is not indicated is treated, care needs to be taken to ensure a reasonable withdrawal period is set. This ensures that no residues of veterinary medicines above the Maximum Residue Limit remain at the time of slaughter or when produce is taken.
Unless the medicine indicates a withdrawal period for the species concerned and at the required concentration, this should not be less than:
- summary of Product Characteristics for any species multiplied by a factor of 1.5
- 14 days, if the product is not authorised for animals producing eggs for human consumption
Milk
- the longest withdrawal period provided in the SPC for any species multiplied by a factor of 1.5
- 7 days, if the product is not authorised for animals producing milk for human consumption
- 1 day, if the product has a zero-hour withdrawal period
Meat and offal from food-producing mammals, poultry and farmed game-birds
- the longest withdrawal period provided in the SPC for meat and offal, multiplied by a factor of 1.5
- 28 days, if the product is not authorised for food-producing animals
- 1 day, if the product has a zero-day withdrawal period
Fish meat
- the longest withdrawal period for any of the aquatic species in the SPC multiplied by a factor of 1.5 and expressed as degree-days
- if the product is authorised for food-producing terrestrial animal species, the longest withdrawal period for any of the food-producing animal species in the SPC multiplied by a factor of 50 and expressed as degree-days
- 25 degree-days if the highest withdrawal period for any animal species is zero
For use in NI
Withdrawal periods apply where the medicine was used, regardless of where the animal is slaughtered.
Eggs
- the longest withdrawal period provided in the SPC for any species multiplied by a factor of 1.5
- 10 days, if the product is not authorised for animals producing eggs for human consumption
Milk
- the longest withdrawal period provided in the SPC for any species multiplied by a factor of 1.5
- 7 days, if the product is not authorised for animals producing milk for human consumption
- 1 day, if the product has a zero-hour withdrawal period
Meat and offal from food-producing mammals, poultry and farmed game-birds
- the longest withdrawal period provided in the SPC for meat and offal, multiplied by a factor of 1.5
- 28 days, if the product is not authorised for food-producing animals
- 1 day, if the product has a zero-day withdrawal period
Fish meat
- the longest withdrawal period for any of the aquatic species in the SPC multiplied by a factor of 1.5 and expressed as degree-days
- if the product is authorised for food-producing terrestrial animal species, the longest withdrawal period for any of the food-producing animal species in the SPC multiplied by a factor of 50 and expressed as degree-days
- 25 degree-days if the highest withdrawal period for any animal species is zero.
If the calculation of a withdrawal period results in a fraction of days, the withdrawal period must be rounded to the nearest number of days, with any half of a day being rounded upwards.
In relation to the calculation of the withdrawal period for milk, if the calculation of the period results in a milk withdrawal period not divisible by 12, the withdrawal period must be rounded up to the nearest multiple of 12 hours.
However, in cases where the authorised withdrawal periods are close to, or longer than, the statutory minimum withdrawal periods, the vet should consider other factors when setting a suitable withdrawal period. Factors to consider include:
- the length of the authorised withdrawal period(s)
- the known pharmacokinetics of the active substance(s) in both the authorised species and the species being treated (if different)
Veterinarians could, for example, increase the authorised withdrawal period by 50%. Where a 30-day withdrawal period is authorised in cattle (meat and offal), a 45-day withdrawal period might be suitable for goats. If a higher dose is given, a longer withdrawal period may be necessary.
Reference: The Veterinary Medicines Regulations 2013, Schedule 4, Point 2.
Find guidance for prescribing vets on the use of the cascade on GOV.UK.
2.6. Livestock Information Service
The Livestock Information Service (LIS), launched in 2022, is used to record movement data for sheep, goats and deer by users in the supply chain, including farmers, markets and slaughterhouses in England.
The LIS enables suppliers to record sheep, goat and deer movements online (LIS-1 and LIS-2 forms), replacing the paper version of the movement document.
The information included in a LIS movement document is the same as in the paper version, including the Food Chain Information (FCI) and the Veterinary Attestation Number (VAN).
The LIS movement document and FCI report should be available for the OV to check and analyse all the relevant information regarding the animals accepted for slaughter.
When mistakes or missing information are identified, the FBO must ensure that the issue is addressed with the supplier as it occurs with the paper versions.
3. Collection and communication of inspection results
In this section
3.1 Introduction
3.1.1 Duty of the FSA
If inspections reveal the presence of any disease or condition that might affect public or animal health or compromise animal welfare the OV is to inform the FBO.
Where the problem arose during primary production, the OV is to inform:
- the farmer
- the farmer’s veterinary surgeon
- where appropriate, APHA
Reference: (EC) 2019/627 Article 39(5).
3.2 Recording of inspection data
3.2.1 Recording of data
The Operations Group team in each establishment should have a system in place to ensure that the results of ante- and post-mortem inspections (AMI and PMI) are recorded accurately and where possible be identified clearly back to the batch of animals (and specifically to the flock / shed for poultry or by slap mark for pigs, as appropriate for the information supplied on the FCI).
The farmer may use this information to improve the health status of his stock. Defra will use the data for disease surveillance therefore the accuracy of the information is vital.
3.2.2 Recording ante- and post-mortem inspection results
All inspection results must be recorded on IRIS2. The data input should be completed in a timely manner. Where possible, this should be on the same day, but it must be completed within 48 hours of slaughter (not including non-operating days). Procedures for data input should be agreed and communicated to each FSA inspection team by the establishment management team.
Note: The CIR 12/1, CIR 12/2 and the PMI 4/8 will still be available for staff to use if required due to local circumstances.
3.2.3 Plants with no IT connection
Where an establishment has no FSA IT connectivity, the FCI, AMI and PMI data is to be collected at that plant and then entered at a later point in time by the MHI or OV when a suitable connection is available.
3.2.4 CCIR for poultry
CCIR plays an important role in meeting the requirements of the Broiler Directive, by reporting where welfare triggers are exceeded, based on conditions observed during ante and post-mortem inspection (welfare indicators).
Where welfare triggers are exceeded, IRIS2 will automatically generate a report. These reports are sent to the SLA and Contracts team and are then checked by a FVL. The SLA and Contracts team then send the individual reports to APHA, with a copy to FSA staff at the relevant establishment.
APHA follow up these reports (for instance, by visiting the relevant farm or requiring an action plan from the producer).
APHA then provide feedback to FSA regarding the outcome of their actions.
Reference: EU Directive 2007/43.
3.3 IRIS2 for all species
3.3.1 IRIS2 application
FSA staff can find guides for IRIS2 in internal files.
3.4 CCIR: electronic feedback
3.4.1 Electronic feedback
Although it is possible for completed inspection reports to be printed from IRIS2, this is discouraged. Electronic feedback should be sent directly from the system to the FBO.
3.4.2 Automated reports
Once the inspection records are marked as complete, the report will be automatically generated and sent via email at 2.30 am (subject to operational need) the following day.
3.4.3 FBO reports
Any FBO wishing to receive the automated reports can do so by supplying a name and email address. FVLs / FVCs, OVs or ITLs should discuss this with the FBO and send the email address(es) to iris@food.gov.uk.
Note: FBOs may have the reports sent to a maximum of 3 email addresses if they wish.
4. Verification and enforcement
In this section
4.1 Verification guidelines for species: FCI full Implementation
Verification guidelines for species: Food chain information full Implementation:
| Process | Responsibility |
|---|---|
| FCI is provided for all animals sent to slaughter. | FBO rearing animals |
| FCI may arrive with the animals (with the exception of poultry, for which it must arrive at least 24 hours in advance) but any item of FCI which might result in serious disruption to the slaughterhouse activity must be received in good time before the animals arrive. | Slaughterhouse FBO |
| The FBO checks the FCI as per HACCP procedures and acts upon it by accepting / rejecting the animals for slaughter. | Slaughterhouse FBO |
| FBO makes FCI available to the OV (OV / MHI if ante-mortem inspection on farm) and notifies them of any anomalies in FCI and of animals that have arrived without this information. | Slaughterhouse FBO |
| Animals are not slaughtered unless FCI is provided, or the OV permits slaughter and carcases and offal are detained until FCI is provided. Note: see section 4.2 on ‘Enforcement’ in this chapter. | OV |
| AM Records: Enter the data for the applicable species on IRIS2 | OV / MHI |
|
PM Records: Enter data on IRIS2 Note: In general (and there may be exceptions to this), a ‘batch’ relates to a group of animals, all of which:
|
OV / MHI |
4.1.1 Rejected meat receipts
The purpose of PMI 4/8 (Rejected Meat Receipt) is to provide a receipt for, and a record of, rejected meat to verify that the FBO has agreed to the voluntary surrender of the meat.
Form PMI 4/8 is no longer required when the inspection data has been entered on IRIS2. If needed a copy of the reports can be printed from IRIS2 and handed to the FBO. The only occasion a PMI 4/8 form should be used is when there is no FSA IT system at the establishment.
Note: In general (and there may be exceptions to this), a ‘batch’ relates to a group of animals, all of which:
- are from the same producer
- and arrive on the same means of transport
- and arrive on the same day
Ensure that a responsible member of plant staff signs the PMI 4/8 and that a copy of the receipt is filed by Operations Group staff. Once signed by the FSA representative and the slaughterhouse FBO, pass the relevant copies of PMI 4/8 to the slaughterhouse FBO.
4.1.2 Recording information on ante / post-mortem form (CCIR)
Information must be accurate. The OV must be satisfied with the system for accurately collecting data in the lairage and at all points on the slaughter line.
4.2 Enforcement
4.2.1 When FCI is not received
FCI must be available for all animals sent for slaughter or, in the case of emergency slaughtered animals or animals slaughtered on the farm, sent for dressing to the abattoir.
FCI must be provided for all animals slaughtered for human consumption, within 24 hours of their arrival.
The OV may permit the animals without FCI to be slaughtered but the health mark must be withheld until the FCI has been provided and examined. Pending final judgement, the carcases and offal must be stored separately from other meat (subject to the provision below).
When animals arrive without FCI but are slaughtered and the meat held pending the arrival of FCI, the meat shall be declared unfit for human consumption and disposed of as an animal by-product if no FCI is provided within this 24-hour period, as required by the Regulations. Refer to Chapter 7 for information on Enforcement.
Note: FCI in cases of on farm emergency slaughter. The Official Health Certificate (GBHC056) that accompanies the bodies of animals subjected to emergency slaughter outside the slaughterhouse, does not contain any of the elements required for FCI, and therefore Food Chain Information must also accompany the body of the animal. This should be completed and signed by the owner of the animal.
4.2.2 Measures in cases of wrong or misleading FCI
If FCI, or any required element(s) of the FCI, is/are wrong or misleading, which has implications for public health and/or animal health or welfare, the OV must inform the FBO and request clarification about this information.
If it is believed that the accompanying records, documentation or other information do not correspond with the true situation on the holding of provenance, or the true condition of the animals, and could mislead the OV, take the following actions:
- the OV must report the issue to the relevant Local Authority (Trading Standards), copying the communication to National Food Crime Unit (NFCU) on foodcrime@food.gov.uk and APHA, when applicable
- the OV must also inform the FSA local veterinary team for awareness
If there is evidence that the FBO at the slaughterhouse knew the information provided in the FCI was inaccurate/false and they were not acting on the information received in good faith, enforcement for obstruction action against the slaughterhouse FBO shall also be taken accordingly.
Reference: Regulation (EU) 2019/627, Article 42.
4.2.3 FCI incomplete
If FCI, or any required element(s) of the FCI set out in Retained (EU) 853/2004, is/are missing, and this has implications for public health and/or animal health or welfare, the OV must inform the FBO and request the missing information.
Where relevant food chain information is not available within 24 hours of an animal's arrival at the slaughterhouse, the official veterinarian shall declare all meat from the animal unfit for human consumption. If the animal has not yet been slaughtered, it shall be killed separately from other animals taking all necessary precautions to safeguard animal and human health.
If any of the legally required FCI details are absent, the normal hierarchy of enforcement should be followed, for example, FBOs that are regularly failing to identify missing parts of the FCI, relying on the OV to identify them to then act.
4.2.4 Circumstances when records indicate that meat must be declared as unfit for human consumption
Where animals are already present at the slaughterhouse, and accompanying records, documentation or other information demonstrates that:
- the animal(s) come from a holding or an area subject to movement prohibition for reasons of animal or public health or
- the rules on the use of veterinary medicinal products have not been complied with or
- other conditions adversely affecting human or animal health are present
The animals must be slaughtered separately and declared unfit for human consumption.
Note: The list of authorised veterinary medicinal products, including withdrawal periods, can be found online via VMD and NOAH. These links can also be found on K2 applications pages in the ‘Link Applications’ listings.
4.2.5 Disposal of meat declared as unfit for human consumption
When the meat cannot be health marked due to absence of FCI or due to the information provided, the meat must be declared unfit for human consumption and the OV should seek voluntary surrender of the meat.
Where surrender is not forthcoming, the OV should put in writing the reasons why they are formally declaring the meat unfit for human consumption, in accordance with (EU) 2019/627, Article 40 and 41.
Note: Where the FBO continues to refuse to dispose of meat declared unfit, follow the ABP provisions relating to the treatment of meat that has been declared unfit for human consumption in chapter 2.8 on ABPs.
5. Annexes
The following documents can be accessed by FSA staff in internal files:
- Annex 1: Cumulative Daily Mortality Rate (CDMR)
- Annex 2: Model document: Letter to FBO permitting slaughter
- Annex 3: Model document: FCI for equines
- Annex 4: IRIS User Guide REMOVED
- Annex 5: IRIS Q&A REMOVED
- Annex 6: IRIS Ante-Mortem Conditions REMOVED
- Annex 7: IRIS Post-Mortem Conditions REMOVED
- Annex 13: FCI Farmed game and fractious bovine slaughtered on farm
- Annex 15: FCI Emergency slaughter REMOVED
- Annex 17: FCI Farmed game animals susceptible to bovine TB
Local Authorities should check in the Food Law Code of Practice and on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms.
The following documents are available to view online:
-
Broilers are considered all chickens kept to produce meat for human consumption, including slow growing breeds and organic production.
-
All farms are approved by default, and approval is withdrawn individually in each of the four countries if they fail to meet their national target. In such an event, this would be communicated appropriately to frontline staff.
-
If such a flock is to be slaughtered for human consumption, a Salmonella NCP sample must be taken before slaughter at the timings described under Birds over 81 days old.
-
Flocks may still need to be tested by officials either as part of the annual random survey or if it appears the flock may be positive for a regulated Salmonella.
-
The pre-slaughter testing can be on farm or at hatchery.
Revision log
Published: 28 October 2021
Last updated: 25 February 2026