Consultation pack on proposals for a new framework in England for the regulation of precision bred organisms used for food and animal feed
The purpose of the consultation is to gather stakeholders’ views on proposals for establishing a new framework in England under the Genetic Technology (Precision Breeding) Act 2023.
1. Purpose of consultation
1.1 The purpose of this consultation is for the Food Standards Agency (FSA) to gather stakeholders’ views on proposals for establishing a new framework in England under the Genetic Technology (Precision Breeding) Act 2023 for the regulation of food and animal feed ('feed') produced from Precision Bred Organisms (PBOs) (footnote 1) ('PBOs for food/feed').
2. Consultation audience
2.1 This consultation will be of most interest to:
- consumers
- UK and international food/feed businesses and industry trade bodies
- competent Authorities (UK Local Authorities and Port Health Authorities).
- non-government organisations / Civil Society
- third-party assurance organisations
3. Consultation purpose/subject
3.1 The Genetic Technology (Precision Breeding) Act 2023 ('the Act') received Royal Assent on 23 March 2023. The Act, the substance of which applies in England, contains powers for the Secretary of State to make secondary legislation under Parts 3, 4 and 5 of the Act to make provision for regulating the placing on the market, in England, of PBOs for food/feed. This consultation seeks to collect views from stakeholders and interested parties on the proposals for regulation and the associated impact.
4. How to respond
4.1 Please respond preferably using the online consultation response form. Alternatively, you can respond via e-mail at: precisionbreeding@food.gov.uk
5. Details of consultation
5.1 The Act defines a PBO as a precision bred plant or precision bred animal. (footnote 2) Precision breeding is a process for introducing genetic changes into the DNA of plants or animals. These changes are introduced using techniques of modern biotechnology such as gene editing and are limited to genetic changes equivalent to those that could have been made through traditional breeding methods.
5.2 Further to the Defra consultation on the regulation of genetic technologies carried out in 2021, it was announced in the Queen’s Speech (PDF) on 10 May 2022 that the UK Government’s intention for legislation to be brought forward to '…unlock the potential of new technologies to promote sustainable and efficient farming and food production'. That legislation – The Genetic Technology (Precision Breeding) Bill - was introduced to Parliament on 25 May 2022 ultimately receiving Royal assent on 23 March 2023 as the Genetic Technology (Precision Breeding) Act 2023.
5.3 This consultation continues the collaborative approach the FSA has taken throughout its precision breeding work programme. It seeks stakeholders’ views on proposals for secondary legislation under Parts 3, 4 and 5 of the Act to establish a regulatory framework in England for PBOs for food/feed. As with the substance of the Act, the secondary legislation will apply to England only, however there will be effects on the other UK nations due to the operation of the UK Internal Market Act 2020 (UKIMA) and the Windsor Framework as described in Section 9 of this consultation document. A glossary of terms can be found at Annex B.
6. Main proposals
6.1 The main proposals presented in this consultation are for a new regulatory framework for PBOs for food/feed, including:
- a pre-market authorisation system designed around the classification of PBOs into two tiers, based on independent scientific advice relating to risk
- a public register of PBOs for food/feed which have received marketing authorisations
- provisions for enforcement of requirements under the new framework
7. Background to proposals
Role of the FSA
7.1 The FSA is a non-ministerial government department with policy remit in England, Wales and Northern Ireland. Our role, as set out in law, is to safeguard public health and protect the interests of consumers in relation to food. In so doing we work closely with, and provide advice to, the UK Government, public bodies and the governments of Wales and Northern Ireland, but we act independently and transparently, led by the latest science and evidence.
7.2 The FSA is led by a Board which sets its strategic direction. Transparency is a guiding principle for the FSA and key to maintaining public confidence and our work is discussed and agreed in public Board meetings. The FSA provides advice to Ministers. The FSA reports to Parliament through the Secretary of State (SoS) and other Ministers at the Department of Health and Social Care (DHSC). Our role in the work on precision breeding is to develop a new regulatory framework for PBOs for food/feed to be presented, informed by responses to this consultation, to the FSA Board and then the Secretary of State for implementation via secondary legislation.
7.3 Since September 2021, the FSA Board has received updates and had opportunities to consider, and provide steers on, issues and proposals in several of its open Board meetings, which are reflected in this consultation. At its September 2021 public meeting the FSA Board agreed a set of principles for the new regulatory framework for PBOs for food/feed focusing on safety, transparency, proportionality, traceability and consumer confidence. These have guided our work throughout the policy development process. Information on FSA Board meetings and public minutes of the meetings can be found on the FSA website.
Key Act powers
7.4 The Act provides powers which will enable the Secretary of State to:
- remove precision bred plants and vertebrate animals from regulatory requirements applicable to the environmental release and marketing of Genetically Modified Organisms (GMOs); (Part 2 of the Act)
- introduce notification processes for PBOs used for both research and marketing purposes and to place relevant information on a public register (maintained by Defra); (Part 2 of the Act)
- establish a regulatory system for precision bred animals to ensure animal welfare is safeguarded; (Part 2 of the Act).
- establish a new pre-market authorisation process for PBOs for food/feed including a public register of PBOs with marketing authorisations maintained by the FSA. (Part 3 of the Act).
- provide for inspection, monitoring and enforcement of provisions made under the Act (Section 20 (Part 2), Section 28 (Part 3) and Part 4 of the Act).
- provide for the payment of fees to an appropriate authority in relation to functions conferred by or under the Act and the and on the issue and the receipt of notices and documents (Sections 39 and 40 (Part 5) of the Act).
Act powers relevant to the FSA
7.5 The powers in the Act, exercisable by the Secretary of State for Health and Social Care, which are relevant to the FSA on the regulation of PBOs for food/feed are in Part 3. Relevant powers relating to enforcement are in Part 3 (Section 28) and Part 4. Relevant powers relating to the payment of fees to an appropriate authority in relation to functions conferred by or under the Act (Section 39) and on the issue and receipt of notices and documents (Section 40) are in Part 5.
7.6 Defra is responsible for the powers in Part 2 of the Act, relating to the requirements placed on precision bred plants and animals before they can be released into the environment for marketing or non-marketing purposes (for example, in research and development trials). Part 2 of the Act includes powers to establish a regulatory system for precision bred animals to ensure animal welfare is safeguarded. Powers in Part 4 (enforcement) and Part 5 (fee and notices) are also relevant.
Policy objectives
Primary policy objectives
7.7 The Court of Justice of the European Union (CJEU) ruled in 2018 that all gene editing techniques were within the scope of EU GM legislation. The UK Government disagreed with this interpretation. The result of this was that companies using gene editing technology to make genetic changes that might otherwise have been achieved using traditional breeding methods would have needed to seek pre-market approval for products under a framework designed to regulate products that have genetic changes which could not have happened using traditional breeding methods. This EU GM law was retained in Great Britain on EU Exit along with CJEU’s legal interpretation as case law. Post EU Exit, the UK Government therefore set about introducing primary legislation to ensure that PBOs are subject to a bespoke legislative regime.
7.8 The primary policy objective of the Act is to ensure that plants and animals developed using precision breeding technologies and food/feed consisting of or containing them are regulated proportionately to risk. The Act provides for simpler regulatory measures to enable these products to be authorised and brought to market more easily through removing PBOs from the GMO requirements and creating a regulatory regime for their use as food/feed with assessment more proportionate to risk.
FSA policy objective
7.9 The FSA’s overall policy objective is to deliver: a proportionate, transparent, regulatory system that will ensure PBOs for food/feed are appropriately assessed for safety before they can be authorised for placing on the market; a public register of authorised PBOs for food/feed; and an appropriate inspection and enforcement regime to ensure compliance with the new requirements. In so doing, our aims are to:
- support innovation in the food system which can bring benefits to consumers;
- provide consumers with assurance via the new regulatory regime and maintain confidence in the food system;
- reduce unnecessary burden on developers in comparison to the current regulatory regime for GMOs.
7.10 Accordingly, we have developed the proposals outlined in this consultation which will inform what will be presented to the FSA Board and then to the Secretary of State for implementation via secondary legislation.
Food labelling
7.11 As there is no scientific evidence that PBOs are intrinsically more hazardous than traditionally bred organisms’ (TBOs) (footnote 3), the UK Government position is that there is no justification for the provision of labelling distinguishing all PB food as such on grounds of consumer safety. As with any food, if there is a need to provide safety information for a particular population group, (for example, hypersensitive consumers or people with certain health conditions) this can be required as appropriate. The UK Government has been clear that there are no plans to require labelling of products to indicate they have been produced using PB techniques. Labelling falls within the policy remit of Defra in England. Labelling was discussed extensively during the passage of the Bill and there is no provision for labelling in the Act. It is therefore not appropriate for us to ask about mandatory labelling in this consultation.
7.12 At its March 2023 public meeting, the FSA Board discussed in public the importance of ensuring that consumers have the right information about authorised PBOs to maintain confidence in the food system and to be able to make informed choices about the food they buy. This discussion was informed by two phases of consumer research published in July 2021 and March 2023. Stakeholders have been updated on developments in the ACNFP’s scientific advice.
7.13 Food businesses will be able to provide consumers with information to inform purchasing decisions in accordance with the terms for voluntary labelling in food information legislation and we expect that some will wish to use this to indicate the benefits of particular products. The FSA will require safety labelling for PBOs for food/feed where this is appropriate for particular consumer groups.
Engagement
7.14 The FSA has worked in partnership with Defra since before their 2021 consultation on the regulation of genetic technologies, through the creation of what is now the Genetic Technology (Precision Breeding) Act 2023 and its passage through Parliament, and more recently in considering how powers under the Act could be exercised to create a proportionate regulatory framework for PBOs. The FSA also engaged with the Department of Health and Social Care (DHSC) through whose ministers the FSA reports to Parliament.
7.15 The regulatory framework proposals in this consultation have been developed through a joint FSA (covering England, Wales and Northern Ireland)/ Food Standards Scotland (FSS) Working Group which provided the mechanism for working in line with the four-nation working principles set out in the Common Frameworks. The Working Group has helped to inform development of, and critically review, the proposals. Given the effects that the PB Act will have on the other UK nations due to the operation of United Kingdom Internal Market Act (UKIMA) and the Windsor Framework (as described in Section 9 of this consultation document), and as a government department with remit in England, Wales and Northern Ireland, there has also been engagement with the Welsh Government and ministers and the Northern Ireland Civil Service.
7.16 We have engaged with non-government stakeholders throughout this process, carrying out a number of stakeholder workshops across three phases as well as ad hoc engagement. In the first phase (August/September 2022) we conducted workshops with UK supply chain, consumer interest, civil society stakeholders and Northern Ireland-specific stakeholders in which we presented our thinking on possibilities for a new regulatory framework. In the second phase (January 2023), we conducted thematic policy workshops with these stakeholders with a particular focus on consumer information and traceability. In the third phase (April 2023) we conducted workshops on enforcement, the application and authorisation process and the public register with UK civil society, industry and consumer interest stakeholders and Northern Ireland-specific stakeholders.
7.17 In developing the proposals, we have considered stakeholders’ views and given regard to international approaches to the regulation of PBOs including through targeted engagement with international stakeholders.
7.18 In terms of the science, we have sought independent scientific advice from the Advisory Committee on Novel Foods and Processes (ACNFP) (see from paragraph 8.7 of this consultation document) on which stakeholders have been updated throughout. Our work has also been informed by the two phases of consumer research mentioned in paragraph 7.12 of this consultation document. Stakeholders have been updated on developments in the ACNFP’s scientific advice.
7.19 Since September 2021, the FSA Board has received updates and had opportunities to consider, and provide steers on, issues and proposals in several of its open Board meetings, which are reflected in this consultation. More information on the FSA Board meetings and the public minutes can be found on the FSA website.
8. Detailed proposals
Overview
8.1 The proposals presented in this consultation are for a new regulatory framework for pre-market authorisation of PBOs for food/feed including:
- a pre-market authorisation system, based on independent scientific advice, designed around the classification of PBOs into two tiers.
- a public register of PBOs for food/feed which have received marketing authorisations.
- provisions for enforcement of requirements under the new framework.
Parameters
8.2 Given the powers in the Act and the parameters within which they are exercisable by the Secretary of State, the breadth of possible options is limited. The basic options are effectively to do nothing (the baseline against which all impact is measured) or to introduce a pre-market authorisation system (or elements of one) as envisaged during the passage of the Bill through Parliament and provided for by the Act.
8.3 The starting point for developing the proposals for a regulatory framework for PBOs for food/feed was the wider regulatory context and legislation which applies by default to all food/feed and therefore will apply to PBOs for food/feed. This includes the existing protection afforded by General Food Law, which provides the foundations for the regulation of all food/feed, including its safety and traceability.
Developing the proposals
Approach
8.4 In developing our proposals, the FSA has also considered independent scientific advice from the ACNFP; FSA-commissioned consumer research; views provided by industry representatives, trade bodies, academic institutions and consumer bodies in England, Wales, and Northern Ireland; potential direct and indirect costs for consumers, industry, enforcement officers and the regulator; international approaches to the regulation of organisms; and the need for any new system of regulation to be future-proofed. The ACNFP’s scientific advice is discussed in detail from paragraph 8.7 of this consultation document.
Wider reform context
8.5 As part of the FSA’s ongoing work on wider regulatory reform, we are considering options to streamline the authorisation processes for regulated products in relation to which there are currently twelve regimes. This is because some regimes are felt to be disproportionately burdensome on both applicants and those involved in operating them and there is potential to achieve the aims of these regimes in a more proportionate way without compromising consumer safety or confidence.
8.6 In designing our proposed approach to the pre-market authorisation of PBOs, we have borne in mind insights from the wider reform work to date.
Pre-market authorisation system for PBOs for food/feed
Independent scientific advice from the ACNFP
Introduction
8.7 The FSA commissioned the ACNFP to provide advice on the current scientific understanding of the technologies used in precision breeding to support the development of a regulatory approach. The objective was to provide the ability to review the full scope of PBOs for food/feed that could be created using the technology in a proportionate way by assigning them to tiers using scientific criteria. The ACNFP sub-committee dedicated to Products of Genetic Technology (PGT) carried out this work, reporting to the main ACNFP committee, which subsequently released three statements on the work.
Overall risk / tiering
8.8 In its first statement (September 2022) the ACNFP advised that that they had seen ‘no evidence that PBOs are intrinsically more hazardous than TBOs’. However, they also recognised that a range of outcomes are possible from this rapidly developing technology and therefore recommended a two-tier approach. This provides a mechanism that allows proportionate scrutiny of the safety of PBOs for consumers and caters for further assessment of any PBOs that raise concerns based on existing evidence or significant uncertainties with regard to their safety.
Triage questions / tier determination
8.9 In its second statement (January 2023) the ACNFP reiterated its support for a two-tier system to allow a proportionate review of PBOs where food or feed safety could be impacted. ACNFP recognised that PBOs contain changes equivalent to those which could have happened through traditional breeding and for many the safety implications are understood. They identified triage questions for all PBOs proposed for food/feed to determine whether further scrutiny is required or not and hence which tier a PBO should fall within.
8.10 The triage questions focus on the following:
- Novelty – whether the PBO would otherwise require assessment as a novel food.
- Composition – whether there is a significant change in composition that impacts the food/feed safety risks in relation to toxicity, nutritional quality or allergenicity.
- Other safety concerns – whether it is intended to provide a route for further review in [rare] cases where there is significant uncertainty around the impact on safety for a PBO, outside the considerations around novelty and composition detailed above.
8.11 Where answers to the triage questions indicate that further scrutiny is required a PBO would be classified as Tier 2. Other, low risk, PBOs would be classified as Tier 1. The two tiers can be described as follows:
- Tier 1: PBOs that are very similar to TBOs for which potential safety risks are understood, do not warrant a bespoke safety assessment and for which there would be a simpler route to market.
- Tier 2: PBOs with traits where further analysis of the data is required. Specifically, this would include novelty or PBOs that have compositional changes which could affect toxicity, allergenicity, nutritional quality or other safety concerns where potential food and feed safety risks need further consideration. For these PBOs there will be a bespoke safety assessment process, including a more detailed examination of the characteristics of the PBO.
Data requirements
8.12 In its third statement (July 2023, published September 2023) the ACNFP considered data requirements – the data which should be requested by the FSA to support the safety assessment of PBOs for food and feed – and proposed two potential models for the FSA’s consideration.
8.13 In considering data requirements and proposing two potential models (hereafter referred to as ‘Model 1’ and ‘Model 2’), the ACNFP noted in its third statement that the models are ‘intermediate points’ on a sliding scale of possible evidence requirements. These range from very extensive to very limited, and the decision about which model to recommend to Ministers would rest with the FSA Board, taking into account what is considered proportionate to manage any risk, alongside consideration of other factors.
8.14 Both Model 1 and Model 2 would require information about:
- The nature and purpose of the genetic change.
- The methods used to make the change.
- The analysis or procedures undertaken to minimise the potential for unintended alteration of the organism’s genetic material (so-called ‘off-target effects’).
- Identification of parts intended for use as food and feed and intended uses.
- The history of safe use for food and feed of the relevant species.
- The predicted impact of change on composition and allergenicity.
- Consideration of known hazards for the species that are managed by developers as part of due diligence; for example, antinutritional factors, toxins, and allergens.
8.15 The main difference between the Model 1 and Model 2 relates to the amount of compositional data considered at the triage stage to determine tier status, and can be described as follows:
- Model 1: A largely descriptive assessment of potential safety risks associated with the intended genetic change, which would require initial data to ensure the intended compositional change (where relevant to the quality or safety of food/feed) has been achieved. Information on how the intended change has occurred and the likely occurrence of food/feed safety concerns would be required.
- Model 2: In addition to the information and data required in Model 1, additional routine data on composition would also be required (e.g. for nutrients/anti-nutrients, toxicology, allergenicity as necessary). This would provide a higher level of assurance of safety.
8.16 In terms of the compositional data required to verify the desired change in a PBO’s physical characteristics where the quality of food and feed is impacted, the data requirements under Model 1 would be less than there would be under Model 2.
FSA Board consideration (September 2023)
8.17 The FSA Board considered a number of potential options for pre-market authorisation based on the ACNFP’s advice at its public meeting on 20 September 2023 and agreed which proposals best met its principles and hence should be presented in this consultation. The FSA considers that the proposed regulatory system set out in this consultation is the one which most closely aligns with the overall policy objectives and the principles for developing the new framework agreed by the Board mentioned in paragraph 7.2.
8.18 The Board agreed that Tier 1 PBOs are sufficiently similar to TBOs so as not to be a concern for consumers as there is not currently any evidence suggesting PBOs pose any more risk than their traditionally bred counterparts and do not warrant bespoke risk assessments. However, the Board agreed that it is appropriate for additional scrutiny in the form of a bespoke risk assessment to be carried out for Tier 2 PBOs on the basis that there could be additional safety factors to consider.
8.19 All developers would need to produce baseline information about their PBOs required by Model 1. The Board was satisfied that Model 1 data requirements will enable the consideration of risk that is necessary at the triage stage for both Tier 1 and Tier 2 PBOs and did not consider it proportionate to mandate developers to produce and maintain the additional evidence for Tier 1 PBOs required under Model 2. The Board was clear that this should be contingent on the FSA having the ability to require data from developers to facilitate checks to ensure that Tier 1 PBOs have been appropriately classified.
Proposed pre-market authorisation system
8.20 The FSA’s proposed approach to the regulation of PBOs for food/feed is a pre-market authorisation system, the framework for which will require secondary legislation. It is proposed that there should be a two-tier regulatory approach to understanding and managing any risks presented by PBOs for food/feed based on the classifications proposed by the ACNFP and using the data requirements for tier determination in their proposed Model 1.
8.21 We are proposing this approach as it meets the policy intention of creating a proportionate regulatory framework that ensures the safety of these products whilst not placing undue regulatory burden on responsible businesses.
8.22 A PBO for food/feed will only be considered under the proposed framework if its PBO status has already been determined by the Defra Secretary of State informed by the advice of the Advisory Committee on Releases to the Environment (ACRE), or if it is the qualifying progeny of a marketable PBO. (There will be a public register of all organisms confirmed as PBOs maintained by Defra).
8.23 PBOs for food/feed will be categorised as falling within either of two tiers, based on scientific criteria proposed by the ACNFP. There will be a different approach for each tier. Information on all PBOs which receive a marketing authorisation (regardless of Tier) for food/feed use will be placed on a public register, maintained by the FSA.
8.24 Based on our consumer research the FSA Board recognised that consumers wanted greater reassurance about the regulatory framework for precision breeding in animals than in plants. Pending Defra’s forthcoming animal welfare declaration process and legislation, our starting position will be to assume an FSA assessment for precision bred animals for food/feed. We will work with Defra to ensure that their Animal Welfare Declaration and our authorisation process together give consumers the reassurance they desire.
8.25 The FSA proposes to adopt the data requirements and criteria for determining the tier status of PBOs at triage stage of the ACNFP’s proposed Model 1.
8.26 Tier 1 PBOs for food/feed would require notification to the FSA. At the triage stage, developers would apply the ACNFP criteria and notify the FSA of PBOs for food/feed falling within Tier 1 according to those criteria. The FSA would acknowledge receipt of the notification and recommend to the Secretary of State that the PBO be authorised for use in food and feed (see paragraphs 8.38 and 8.39 of this consultation document on decision-making). Developers of a Tier 1 PBO granted a food/feed marketing authorisation would receive a communication from the FSA to that effect and the FSA would place the PBO on the public register.
8.27 Tier 2 PBOs for food/feed would require an application to the FSA as with other regulated products. At the triage stage, developers would apply the ACNFP criteria and in respect of PBOs for food/feed falling within Tier 2 would be required to submit an application with:
- data as required by the ACNFP’s proposed Model 1 demonstrating how Tier 2 status was determined;
- any additionally identified data which may be required for bespoke risk assessment relevant to the factors in respect of which the PBO was determined as Tier 2 (i.e. novelty, composition or other concerns).
8.28 The FSA would then carry out a bespoke risk assessment followed by risk management in accordance with its risk analysis process. Following completion of this process the FSA would recommend to the Secretary of State that the PBO should be authorised (with or without conditions of use) or not authorised for use in food/feed (see paragraphs 8.38 and 8.39 of this consultation document on decision-making). Developers of a Tier 2 PBO granted a food/feed marketing authorisation would receive a communication from the FSA to that effect and the FSA would place the PBO on the public register.
8.29 A diagram showing the pathways for the pre-market authorisation of PBOs for food/feed can be found at Annex A.
Guidance
8.30 The FSA will produce administrative and technical guidance for the operation of the process. We will develop and, following engagement with stakeholders publish, technical guidance that will outline what the data requirements are for the triage of Tier 1 and Tier 2 PBOs in a format that supports self-determination by developers; while the additional data which may be required for each factor by which a PBO may be determined as Tier 2 (i.e. novelty, composition or other concerns) will be bespoke, the guidance may identify those that are predictable.
Transparency
8.31 Section 26(4) of the Act provides for secondary legislation concerning the publication of information on applications for marketing authorisations. As PBOs for food/feed will be a 'regulated product', interested parties will be able to view notifications (Tier 1) and applications (Tier 2) on the public Register of Regulated Product Applications on the FSA website. Notifications / applications for PBOs for food/feed will be managed and trackable in our Case Management System, enabling developers to see what is progressing though the system at any given time. PBOs for food and feed that have received marketing authorisations will be entered on a public register maintained by the FSA (see from paragraph 8.48 of this consultation document).
Checks and balances
8.32 At the September 2023 FSA Board meeting (see paragraphs 8.17-8.19 of this consultation document), the Board asked officials to provide more detail about how the FSA can check that businesses have correctly identified Tier 1 PBOs for food/feed and properly considered the evidence. We will establish a pre-market audit system to provide additional assurance/transparency in respect of Tier 1 PBOs. This process will allow the FSA to monitor the effectiveness of its guidance in helping developers determine tier status; help us understand where any changes might be necessary to improve the regulatory framework and/or associated guidance and would act to deter developers from non-compliance with the triage process (which would trigger penalties).
8.33 The notification of a Tier 1 PBO could therefore involve the FSA requiring data specified by ACNFP in Model 1 to be provided either:
- at the point of notification, or
- on request after notification, or
- on request after authorisation.
8.34 The pre-market audit process will be adaptable with regard to:
- the volume/proportion of notifications selected for audit;
- the frequency of audit/how often audit is conducted;
- the stage in the process at which audit is conducted.
8.35 The FSA will initially make decisions on how to determine these factors and which notifications are selected for audit. The results of audit will then be used to optimise these factors to ensure audit stays relevant, informative and proportionate. In time this will dynamically move towards targeted audit in the light of experience, intelligence, levels of compliance and/or further scientific advice.
8.36 This continuous improvement approach to audit will ensure the FSA can continue to provide the right level of assurance to consumers in the most efficient way, as well as providing adequate support for businesses new to the regulatory process. Monitoring compliance in this way will also generate evidence of the degree to which business is correctly determining the tier status of PBOs against which the level of audit controls could be calibrated.
8.37 For Tier 2 applications the FSA would require the data specified by ACNFP in Model 1 used to determine tier status to be provided at the point of application (together with the other data required carry out the bespoke risk assessment as well as additional data companies may wish to use to support their application).
Decision-making / giving legal effect to marketing authorisations
8.38 The Act does not prescribe the use of secondary legislation to grant PBOs marketing authorisations. As such a more streamlined process is proposed, as mentioned above, where marketing authorisations for PBOs for food/feed will be subject to a decision by the Secretary of State. Where authorisation is granted, a PBO will then be entered on a public register maintained by the FSA (see from paragraph 8.48 of this consultation document).
8.39 This process would help to significantly reduce the authorisation timeline compared to other regulated products regimes under which authorisations require secondary legislation, maintaining consumer trust and consumer safety whilst reducing unnecessary burdens on those operating the regime and businesses.
Conditions for issuing a marketing authorisation
8.40 Section 26(3) of the Act provides for secondary legislation to stipulate requirements which must be satisfied for a marketing authorisation for PBOs for food/feed to be issued. The FSA proposes that secondary legislation will stipulate that the following conditions must all be satisfied:
- the product proposed for pre-market authorisation has been confirmed to be a PBO by the Defra Secretary of State (or is the qualifying progeny of a confirmed PBO);
- the food/feed produced from the PBO under the authorisation will have no adverse effects on human or animal health;
- the way in which the food/feed is marketed will not mislead consumers;
- the production of the food/feed will not have adverse effects on the environment; and
- consuming the food/feed in place of food/feed it might reasonably be expected to replace will not be nutritionally disadvantageous to humans or animals.
Marketing authorisations: procedure etc
8.41 Section 26(4) of the Act provides for secondary legislation concerning the procedure for determining applications for marketing authorisations for PBOS for food/feed. The FSA proposes that secondary legislation will provide powers for the Secretary of State for Health and Social Care to determine applications / notifications and set down a procedure for this based on the proposed approach set out in this consultation.
8.42 Section 26(4) also provides for authorisations to be issued subject to conditions/limitations; for varying or cancelling such conditions/limitations or imposing new ones; and for the revocation of authorisations. The FSA proposes that secondary legislation will provide powers for the Secretary of State to take such action where it is appropriate to do so.
Four-nation working
8.43 Pre-market authorisations for PBOs for food/feed will apply to England only. However, we are mindful of the operation of the UKIMA and Windsor Framework (as described in Section 9 of this consultation document), the FSA/FSS Risk Analysis principles/arrangements and commitments to four-nation working under the Common Frameworks. As such we will apply a four-nation approach (FSA in respect of England, Wales and Northern Ireland and FSS in respect of Scotland) to ensure that the other UK countries are aware of the PBOs for food/feed being considered for placing on the market in England, to ensure full transparency across the UK.
Obligations on food/feed business operators
8.44 The FSA proposes that secondary legislation under Section 26 of the Act should prohibit the placing on the market of food/feed from PBOs except in accordance with a marketing authorisation for that PBO. It will also require compliance with:
- any conditions of a marketing authorisation relating to a PBO for food/feed
- any requirement imposed by an inspector in exercising their function
- any requirement to provide or record information
8.45 Under Section 29 of the Act these are considered to be ‘Part 3 obligations’. Section 29 stipulates that Part 3 obligations also include any requirement that is imposed by a compliance notice or stop notice (see paragraph 8.79 of this consultation document). The FSA proposes that the secondary legislation will also require that information provided or recorded, or any statement made in purported compliance with Part 3 obligations or in connection with marketing authorisations, is not false or misleading. This requirement is also considered to be a Part 3 obligation under Section 29.
8.46 Information on proposals around the enforcement of the new regulatory regime including defences to breaches can be found from paragraph 8.69 of this consultation document.
Fees
8.47 Section 39 of the Act provides for secondary legislation to require the payment of a fee to the FSA in respect of the exercise of any function conferred on it by, or under, Part 3 of the Act, and must set out the amount of the fee or how it is to be calculated. The Act requires that income from such fees is on average equal to expenditure incurred by the FSA in exercising relevant functions (including a reasonable share of expenditure partly or indirectly incurred in so doing). The FSA is not proposing that the secondary legislation will introduce fees at this time but will be considering this as part of wider regulated products reform, informed by responses to this consultation.
Consultation question: Pre-market authorisation process
1. Triage and two-tiered system
Tier 1 PBOs: Developers will apply the ACNFP criteria to determine tier and notify the FSA of the PBO status. Tier 1 notification is acknowledged by the FSA. When the authorisation decision is taken by the Secretary of State, the FSA will communicate this to the developer and, if the decision is to authorise the PBO for food/feed, place it on the public register.
a. To what extent do you agree with the FSA using a two-tiered approach for the pre-market authorisation of precision bred organisms used in food and feed? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
b. To what extent do you agree that the proposal for Tier 1 notifications meets the FSA’s policy objectives in paragraph 7.9 of this consultation document? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
c. To what extent do you agree or disagree that the proposal for Tier 1 notifications is feasible? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
d. Please provide details of your thoughts towards the initial audit process for Tier 1 PBOs [Free text].
e. Please provide details of any barriers that may exist which are preventing the policy objective being met or the proposal being implemented [free text]
f. Please provide details of what you think the benefits and disbenefits of this approach are [Free text]
g. If you feel there is anything missing from our proposal which would be required to ensure that the policy objectives can be met, or the proposal can be implemented please provide any additional comments you have on the Tier 1 process here. [Free text]
2. Tier 2 PBOs: These would be subject to an application to the FSA, similar to other regulated products. Developers would apply the ACNFP criteria to determine tier. Developers with PBOs for use in food and feed falling within Tier 2 would be required to submit an application with the accompanying data described in ACNFP’s Model 1. Applications would be subject to a bespoke risk assessment and risk management process. When the authorisation decision is taken by the Secretary of State, the FSA will communicate this to the developer and, if the decision is to authorise the PBO for food/feed, place it on the public register.
a. To what extent do you agree with the FSA conducting bespoke risk assessments for Tier 2 PBOs prior to them being authorised for use in food/feed [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
b. To what extent do you agree that the proposal for Tier 2 applications meets the FSA’s policy objectives in paragraph 7.9 of this consultation document? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
c. To what extent do you agree or disagree that the proposal for Tier 2 applications is feasible? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
d. Please provide details of any barriers that may exist which are preventing the policy objective being met or the proposals being implemented [Free text]
e. Please provide details of what you think the benefits and disbenefits of this approach are [Free text]
If you feel there is anything missing from our proposals which would be required to ensure that the policy objectives can be met, or the proposal can be implemented please provide any additional comments you have on Tier 2 process here [Free text]
Public register of PBOs for food/feed
8.48 The Act provides a discretionary power in Section 27 for the Secretary of State to make regulations to require the FSA to establish and maintain a public register of information relating to PBOs which have been granted marketing authorisation for use as food/feed in England.
8.49 As with other regulated products, the FSA proposes that there should be such a public register available to any interested party including consumers, industry and enforcement authorities and that this should include all PBOs regardless of tier. Our consumer research and wider stakeholder engagement indicates that consumers support the concept of a public register and felt strongly it should exist for transparency and provide assurance that PBOs for food/feed was being regulated effectively, therefore providing a proportionate way to assure people of the safety of these food products. They also indicated that basic information was not sufficient and that information on the purpose of the edit (i.e. why the organism was precision bred) and details of any associated safety assessment should be included in the register.
8.50 We therefore propose that there should be a public register containing the following information for each PBO for food/feed, which includes information requested by consumers:
- Name of the PBO
- Authorisation holder
- Purpose of the edit
- Date of authorisation
- Any conditions of authorisation (e.g. any mandatory product-level information that should be provided)
- A unique reference number (URN) for each authorised PBO. (This could both assist search functionality and enable businesses to include this URN on commercial documentation, should they wish to do so)
- A link to the relevant entry on the Defra register confirming PBO status
- Details of any bespoke safety assessment
Consultation questions: Public register
The Act makes provision for the FSA to establish and maintain a public register which will provide details of PBOs authorised for use in food/feed.
a. To what extent do you agree that the proposal for a public register meets the FSA’s policy objectives in paragraph 7.9 of this consultation document? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
b. Please provide details of what you think the benefits and disbenefits of this approach are [Free text]
c. If you feel there is anything missing from our proposal which would be required to ensure that the policy objectives can be met please provide any additional comments on the Public Register here. [Free text]
Traceability of food/feed from PBOs
Central policy objective
8. 51 The FSA’s central policy objective for the traceability of food/feed from PBOs is to ensure that it can be identified and removed from the market in the event of an incident, taking into account the levels of traceability required to achieve the same objective for TBOs. This approach is being taken due to the lack of evidence that PBOs would be intrinsically riskier to consumers than their traditionally bred counterparts, making the FSA’s objective proportionate.
8.52 Section 26 of the Act provides a power for the Secretary of State to make regulations to impose requirements for the purpose of securing traceability of food/feed from PBOs. As with our approach to pre-market authorisation, the wider regulatory and regulatory reform contexts have been taken into account, with initial consideration given to the legislation and enforcement provisions which are applicable to all food/feed and therefore will apply to all food/feed from PBOs.
Wider legislative context
8.53 Traceability is a fundamental component of food law and existing traceability requirements in General Food Law (footnote 4) provide strong assurance throughout food/feed supply chains. Traceability is the ability to trace and follow food/feed, food-producing animals or substances intended or expected to be incorporated in food/feed, through all stages of production, processing and distribution. Under General Food Law, business operators must be able to identify their immediate suppliers as well as the businesses to which their products are supplied (a 'one up, one down' approach). This information must be provided to enforcement authorities, if requested.
8.54 In addition, whilst the Act and secondary legislation relates to England only, the effects of the UKIMA and the Windsor Framework as outlined in Section 9 of this consultation document present additional challenges and have also been considered.
Testing for PBOs
8.55 Since changes achieved by precision breeding can, by definition, be achieved by traditional breeding, PBOs cannot readily be identified or distinguished from their traditionally bred counterparts through sampling and analysis with a sufficient degree of certainty. This is however not unique to food/feed from PBOs and also arises for example in relation to products of novel processes and organic food, where it is not possible to test to verify its precise status.
8.56 The detectability of PBOs was raised by stakeholders as a possible tool to facilitate enforcement. The FSA explored the possibility of testing and commissioned research by the Government Chemist to identify whether there were analytical methods of detection for PBOs and, if so, which were most suitable.
8.57 The report highlighted that there are no methods of providing unequivocal detection of the genetic change in most PBOs defined by the Act, without prior knowledge of the altered genome sequence and suitable reference materials. For those PBOs where detection may be possible, it is not currently feasible to distinguish whether the genetic changes are the result of genome editing, natural variation, or traditional breeding methods. In cases where detection was possible, this is likely to be lost in subsequent generations.
8.58 The FSA’s response to the report noted that detection cannot currently be guaranteed to a sufficient level of certainty and as such the effectiveness of detection methods as tools for enforcement is limited. We noted that if the data required to establish an appropriate detection method for some PBOs was obtained as part of the authorisation process, this would not enable the unequivocal identification of how the edit was generated.
8.59 We were also mindful of the extra costs for developers and the PB market of requesting additional data beyond that necessary to support safety assessment. There is the potential for this to reduce incentives for food businesses to innovate and bring new products to market, which in turn would minimise potential benefits. We therefore do not currently consider detection to be practical due to the capability and capacity required for delivery or proportionate to the risks. Enforcement bodies would require sufficient intelligence to know what they were looking for, as screening for PBOs is not possible in the same way as it is for GMOs.
8.60 Considering the issues of proportionality and feasibility described above and in pursuing a proportionate approach, the FSA will not be taking forward the report’s recommendations associated with implementing an infrastructure for further development of analytical methods for the detection of PBO products or pursue detection as an enforcement tool at this stage. As a science and evidence-based regulator, however, we would welcome further research in this area in the future to ensure we have the most up to date scientific information available when reviewing policy and/or developing new policies related to genetic technologies.
Practicalities
8.61 For any food and feed supply (including international trade) the supplier must ensure that the products they deal with meet the requirements of the customer, including the legal requirements of the market into which they are selling. This can be achieved through commercial contracts specifying supply chain controls, supported by documentation and audit without the need for additional traceability requirements and, in this instance, would be supported by the FSA’s Public Register of PBOs authorised for use as food/feed.
8.62 Industry and enforcement authorities must already ensure compliance with existing legislation in order that PB products produced in other countries cannot currently enter the UK market where they are not authorised as GMOs and labelled as such. Similarly, food/feed businesses in Great Britain (GB) which send products to Northern Ireland must already ensure that products are compliant with relevant legislation under the terms of the Windsor Framework (see Section 9 of this consultation document).
8.63 The main challenges are less about the ability to trace the food/feed itself and more about whether it would be possible for industry to distinguish, via mandatory traceability information, whether products are suitable for food/feed use for the UK and external markets, and for UK enforcement authorities to determine compliance with relevant rules at any point along the domestic food/feed supply chains. The challenges arise in part because of the inability to readily test for PBOs and in part because they are inherent in the food system and not unique to food/feed from PBOs.
8.64 Similar challenges arise for example in relation to products of novel processes, organic food and to an extent, food labelled with a country of origin, where it is not readily possible to test to verify its status beyond use of assured supply chains and audits of documents and records etc.
8.65 Fraud risk is inherent in the food system and there are additional challenges where there is no test to determine authenticity. Risks around food/feed from PBOs will need to be mitigated by using the similar approaches to detecting and preventing fraud applied to products that cannot readily be identified through testing such as those mentioned above and focused investigations of the supply chain where fraud is suspected.
8.66 Additional requirements for information which distinguishes food/feed from PBOs as such along supply chains would mitigate the challenges to only a limited extent and would only be effective under the powers in the Act in England. It would require the segregation of food/feed from PBOs from its traditionally bred counterparts at all stages requiring significant changes to physical infrastructure as well as new industry systems and processes whose costs are likely to be passed on to consumers. However, limitations around enforcement and industry assurance would remain regardless. This is due to the inability to conduct tests to check for PBOs in food/feed as well as the need to otherwise take the information at face value where an audit of systems and records had not been carried out.
Voluntary approach
8.67 Should there be a consumer market for food/feed which excludes PBOs, food/feed law does not preclude a certification scheme being developed by industry on a voluntary basis. Such a scheme would necessitate a similar approach to that described above involving segregation, albeit on a voluntary basis. It is likely that such food/feed would be more expensive due to this and there would be a cost to bodies associated with administering any such scheme, but it would mean that the costs of distinguishing food/feed from PBOs as such along the supply chain would be incurred to support a specific market rather than being applied to all food/feed from PBOs on a blanket basis. This aligns with our aim of a proportionate regulatory approach which does not place unnecessary costs on consumers who wish to benefit from PBOs or industry.
Proposal
8.68 Balancing all the issues outlined above, the FSA believes that existing General Food and Feed Law traceability requirements are proportionate and sufficient to support our central policy objective and that they will allow business to work with existing familiar requirements without creating any further burden. The FSA therefore proposes that no traceability requirements for food/feed from PBOs beyond those in General Food Law are adopted at this time. Enforcement officers will be able to use information obtained from the audit of systems, records and paperwork to ensure that only authorised PBOs are marketed for sale, in addition to the FSA’s Public Register of PBOs authorised for food/feed. Furthermore, this would not prevent approaches such as independent certification schemes for food/feed which excludes PBOs being adopted, should there be a viable market.
Consultation questions: Traceability
In relation to traceability the proposal is that no requirements beyond the existing traceability provisions in General Food Law which apply to all food and feed are necessary.
a. To what extent do you agree or disagree that the proposal to use existing provisions in General Food Law for traceability meets the FSA’s policy objectives in paragraph 7.9 of this consultation document? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
b. Please provide details of any barriers that may exist which are preventing the policy objective being met or the proposal being implemented [Free text]
c. Please provide details of what you think the benefits and disbenefits of this approach are [Free text]
d. If you feel there is anything missing from our proposal which would be required to ensure that the policy objectives can be met, or the proposal can be implemented please provide any additional comments you have on Traceability here. [Free text]
Enforcement, sanctions and defences (England)
Introduction
8.69 As part of the proposed new regulatory framework for food/feed from PBOs, the FSA is responsible for developing the approach to enforcement by Local Authorities and Port Health Authorities ('enforcement authorities') in England, including the provision of powers and sanctions. Our aim is to develop a proportionate enforcement regime which will enable these enforcement authorities to take effective action against non-compliance and which maintains consumer confidence.
8.70 As with other aspects of the proposed regime, the starting point for developing proportionate proposals relating to enforcement was to consider the wider regulatory context and the existing legislation and enforcement provisions which are applicable to all food/feed and therefore will apply to food/feed from PBOs.
8.71 We have considered what additional provisions, provided for by the Act, are necessary to complement existing food/feed law enforcement powers in England. In developing our proposals, we have also considered the fact that it is not possible to readily carry out sampling/analysis which will identify whether a food/feed is, or contains, PBOs.
Enforcement context
8.72 Enforcement authorities will use the same approaches for detecting and preventing fraud and minimising the risk of inaccurate information passed along the supply chain as they do for other products that cannot readily be identified through testing, such as organic food.
8.73 This will be via audits of food and feed business systems and records and focused investigations on the supply chain where non-compliance is suspected. In so doing, enforcement authorities will be able to use traceability information required under General Food Law, information obtained from the audit of systems, records and physical/digital documents, to help ensure that only authorised PBOs are marketed for sale under existing traceability requirements under General food law. They will also be able to use a public precision breeding register maintained by Defra under Section 18 of the Act and the public register of PBOs authorised for use in food/feed maintained by the FSA (see from paragraph 8.48 of this consultation document) as an additional supporting resource.
Restrictions on sanctions
8.74 Section 28(4) of the Act specifies that criminal sanctions are not permitted but provides for civil sanctions which the FSA considers proportionate to the risk posed by breaches of requirements relating to food/feed from PBOs. As such, regulatory breaches would need to be addressed by enforcement officers without recourse to the courts, reducing the cost and resource needed to take formal action to address non-compliance and avoid burden on the justice system. Details of these sanctions can be found in paragraph 8.79 of this consultation document.
‘Part 3 obligation’ / ‘relevant breach’
8.75 Paragraphs 8.44-8.46 of this consultation document indicate that secondary legislation establishing the new regulatory framework for food/feed will place obligations on food/feed business operators (‘Part 3 obligations’) and provide information on these. Section 31 of the Act states that a ‘relevant breach’ includes the failure of a person to comply with a Part 3 obligation and that the following matters can be treated as a relevant breach:
- where a food and feed marketing authorisation is subject to conditions, the obligation to comply with those conditions;
- any requirement imposed by an inspector in exercise of the inspector’s functions.
Application of the Food Safety Act 1990
8.76 Section 28(f) of the Act provides that secondary legislation may make provision corresponding to, or applying (with or without modifications), any provision made by or under the Food Safety Act 1990 (or Section 67 of the Agriculture Act 1970). The FSA proposes that Food Safety Act 1990 provisions are applied where appropriate in secondary legislation providing functions and powers to enforcement authorities as outlined in this consultation.
Powers for enforcement authorities and inspectors
8.77 Section 28 of the Act provides powers for the Secretary of State to make secondary legislation to designate public bodies as enforcement authorities, provide powers for enforcement authorities to appoint inspectors and to confer functions on and provide enforcement powers for inspectors.
8.78 Accordingly, the FSA proposes that secondary legislation will designate Local Authorities and Port Health Authorities in England as enforcement authorities for the new regulatory framework for food/feed from PBOs and provide the following enforcement functions and powers to inspectors to enable them to monitor compliance and investigate suspected failures to comply with the requirements of the framework:
- Powers for enforcement authorities to appoint inspectors to carry out enforcement functions.
- Powers enabling inspectors to investigate potential breaches, specifically:
- powers of entry, (footnote 5) inspection, examination, search and seizure;
- powers to take copies of documents, photographs and samples;
- powers to impose requirements; and
- powers to require the provision of information.
- Providing for inspectors to be accompanied by other persons in carrying out their functions.
- Providing relief for inspectors from criminal or civil liability for acts done in good faith in the purported exercise of their functions.
Sanctions
8.79 The secondary legislation will also provide powers for inspectors to issue the following enforcement notices to compel specific actions and set out conditions for their use and their content including, for example, grounds of issue, rights to require a review or appeal and consequences of a failure to comply:
- Compliance Notice: Requires a person to take specified steps within a specified period of time. (Section 33 of the Act)
- Stop Notice: Prohibits a person from carrying on a specific activity or from doing so until specified steps have been taken. Enforceable by injunction. (Section 34 of the Act)
- Monetary Penalty Notice: In relation to a relevant breach (see paragraph 8.75), requires a person to pay a sum specified on a notice to the enforcement authority, on which interest may be charged for late payment. (Section 35 of the Act).
- Costs Notice: Requires a person to pay costs incurred by an enforcement authority in relation to an enforcement notice up to the point it is issued (e.g., costs of investigation, administration and obtaining expert, including legal, advice). (Section 38 of the Act)
8.80 In order to underpin the enforcement provisions, the FSA proposes that secondary legislation will also provide that the following may be treated as a relevant breach:
- Obstructing an inspector.
- Providing false information to an inspector.
- Impersonating an inspector.
- Providing or recording information, or making a statement, that is false or misleading: in purported compliance with a Part 3 obligation; in connection with any proposal to suspend or revoke an authorisation; or any proposal to vary, revoke or impose conditions or limitations on an authorisation.
Monetary penalty notices
8.81 The Act provides for monetary penalty notices to be issued for 'relevant breaches' as described above. Penalties could be a set sum (or set range), a percentage of a developer/food business operator’s turnover or a mixture. Penalties will need to be set at a level that ensures this tool is an effective deterrent to non-compliance. The responses to this consultation will inform our consideration. Please see the consultation question below about monetary penalties.
Issue of notices and documents
8.82 The FSA proposes that the secondary legislation will also set out how notices and documents are issued or given under the Act and when they are treated as having been received.
Defences
8.83 The FSA proposes that secondary legislation under Section 31(3) of the Act will provide for the following defences:
- Offences due to the fault of another person: Persons to be treated as failing to comply with a Part 3 obligation in circumstances in which Section 20 of the Food Safety Act 1990 provide for such persons to be guilty of an offence.
- For failure to comply with a Part 3 obligation not to be treated as a relevant breach:
- In circumstances in which Section 21 of the Food Safety Act 1990 provides for a defence to offences under that Act (Due Diligence).
- For circumstances in which Section 22 of the Food Safety Act 1990 provides for a defence to offences under that Act in respect of the advertisement for sale of any food (Publication in the course of business).
Reviews and appeals
Reviews
8.84 The FSA proposes that secondary legislation under Sections 37 and 38 of the Act will provide for a person to whom an enforcement notice or costs notice is issued:
- to require the Secretary of State to review the decision to issue the notice (in accordance with any provisions set down for the Secretary of State’s exercise of such functions)
- to appeal against the decision if not satisfied with the outcome of a review
8.85 The secondary legislation will set out the grounds on which a review may be required i.e. that it was based on error of fact, was wrong in law, or the requirements of the notice were otherwise unreasonable. In respect of a stop notice a further ground will be that the person had not and would not have committed a breach. In respect of a monetary penalty notice a further ground is that the monetary penalty is itself unreasonable.
Appeals
8.86 The FSA proposes that secondary legislation under Sections 37 and 38 of the Act will provide for appeals against a review decision to be brought in First-tier Tribunal, set out the grounds for appeal and provide for suspending the operation of a compliance notice, monetary penalty notice or costs notice pending the outcome of an appeal. It will also provide powers of the First-tier tribunal in relation to an appeal under Section 37.
Consultation questions: Enforcement (England)
As part of the proposed regulatory framework for food/feed from PBOs, the FSA is proposing enforcement powers and tools for Local Authorities and Port Health Authorities ('enforcement authorities') in England. The Act does not allow for criminal sanctions beyond those available in existing food/feed law which may be used in respect of food/feed consisting or containing PBOs where appropriate.
a. To what extent do you agree or disagree that the proposed enforcement regime meets the FSA’s policy objectives in paragraph 7.9 of this consultation document? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
b. To what extent do you agree or disagree that the elements of the proposed enforcement regime are practical and deliverable? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
c. To what extent do you agree that this proposal meets your need as a stakeholder? [Strongly agree/Agree/Neutral or Don’t know/Disagree/Strongly disagree]
d. Please provide details of any barriers that may exist which are preventing the policy objective being met or the proposal being implemented [Free text]
e. Please provide details of what you think the benefits and disbenefits of this approach are [Free text]
f. What level(s) of monetary penalty do you think would be appropriate in respect of the 'relevant breaches' outlined in the consultation document?
g. If you feel there anything missing from our proposals which would be required to ensure that the policy objectives can be met, or the proposal can be implemented please provide any additional comments you have on Enforcement here. [Free text]
9. Regulatory implications for PB food/feed moving within the UK
9.1 The Act applies to England only. However, under UKIMA market access principles, food/feed from PBOs which have a marketing authorisation in relation to England which has been produced in, or imported into, England could also be sold lawfully in Wales and Scotland. This would be the case even if the PBOs were not authorised for use in food and feed under existing GMO legislation relating to those countries which would continue to apply.
9.2 The UKIMA does not apply to processing after sale if this constitutes a 'significant production step' for the purposes of the UKIMA. As such, any food/feed from PBOs which is sold in Wales or Scotland under UKIMA market access principles which then undergoes a significant production step there after sale is considered under the UKIMA to have been produced in that nation and as such would be subject to legislation regulating GMOs. In order for the processed product to be placed on the market in those countries, it would need to be authorised and labelled in accordance with GMO legislation or otherwise sent to a third country provided it meets its relevant domestic law. The Welsh and Scottish Governments retain powers to legislate in this devolved policy area should they wish to.
9.3 In relation to Northern Ireland, the provisions of the Windsor Framework will apply. GB public health and consumer protection standards apply for all prepacked retail food moving from GB to Northern Ireland via the Northern Ireland Retail Movement Scheme (‘green lane’). This ensures that Northern Ireland consumers have access to the same goods as the rest of the UK. All goods moved from GB into Northern Ireland which are destined to move onwards into the EU single market will move via the ‘red lane’ and must meet all EU rules. The Scheme will extend to some prepacked retail pet food and dog chews. However, feed from PBOs, whether for feeding directly to livestock or being used to produce compound feeds would not qualify for the scheme. These goods must move via the ‘red lane’. Qualifying Northern Ireland goods will continue to have unfettered market access to GB.
10. Regulatory implications for trade
10.1 Imports, including from the EU, must continue to meet relevant GB standards to be lawfully placed on the GB market. PBOs from other countries must meet the regulatory requirements for PB food/feed to be imported into England. Goods imported directly into Wales or Scotland would need to comply with relevant legislation in those countries whose GMO regulations will continue to apply to PB food/feed. Imports directly into Northern Ireland must meet EU rules. Food exported from the UK to other countries/blocs will need to continue to meet the rules of those countries/blocs.
11. Assessment of impact
Introduction
11.1 The FSA’s assessment of the impact of the proposed regulatory framework for PBOs estimates an impact below the minimum threshold of +/- £10m (PDF). As such a full impact assessment has not been prepared.
11.2 However, in line with the obligation to assess the impact of policy proposals, we have assessed the impact of those set out in this consultation as below. The impact has been assessed against the status quo - a baseline of the existing GMO legislation under which PBOs would currently require pre-market authorisation and is set out alongside the detail of the proposals.
11.3 This assessment only considers direct impacts from the new regulatory framework. This is to avoid the risk of double counting the costs and benefits that have already been accounted for within Defra’s IA on The Genetic Technology (Precision Breeding) Bill (PDF). Moreover, materialisation of the impacts assessed by Defra and the FSA will be contingent on the policy being fully implemented.
11.4 Whilst the Act and proposals in this consultation relate to England, there are also indirect effects on the other countries of the UK as a consequence of other legislation as outlined in Section 9 of this consultation document.
Impacts
11.5 The proposal set out in this consultation has been assessed and appraised against the status quo – do nothing (PBOs for food/feed continue to be regulated as Novel Foods). Overall, the impact of implementing a new regulatory framework is expected to impose minimum burden on business, public/ enforcement bodies and consumers.
Main affected groups
A: Business
Costs
Pre-market authorisation process
11.6 Familiarisation cost (one-off): The main direct one-off cost to business will be familiarisation with the new regulatory framework via technical guidance and FSA guidance. The cost of this has been calculated on the basis of the following estimates/assumptions:
- it would take one legal professional per plant breeding business 2 hours and 15 minutes to read and familiarise themselves with the guidance;
- the median hourly rate of £28.08 (footnote 6) for a legal professional;
- 75 commercial plant breeding companies in the UK will be affected by the proposal (consistent with Defra’s Impact Assessment (footnote 7)).
11.7 To calculate the total cost, the average cost per business of £63 (familiarisation time of 2 hours and 15 minutes multiplied by the uplifted wage of a legal professional of £28.08 per hour) is to be multiplied by the total number (75) of commercial plant breeding companies. This gives a total one-off familiarisation cost of approximately £4,700.
11.8 Self-assessment of tier status (ongoing): Businesses will need to follow the ACNFP triage criteria to self-assess their tier status. The cost is expected to be minimal given developers are expected to have the information readily to hand, and given it is a commercial decision to enter the PB market, these costs are not in scope of this consultation.
Public Register of PBOs for Food/Feed
11.9 No additional costs incurred to business. The FSA will be fully responsible for the public register.
Provisions for enforcement of requirements under new framework
11.10 There will be no additional cost to businesses. All familiarisation costs are already captured under the pre-market authorisation process, where businesses will have already familiarised with the new regulatory framework including provisions for enforcement requirements via the aforementioned technical and FSA guidance.
Benefits
11.11 By reducing regulatory burdens, the new regulatory framework can help simplify and streamline the process by which food businesses can bring PBOs to the market. Food businesses are also able to innovate and bring new products derived from genetic technologies to market. However, at this stage of the assessment it has not been possible to quantify the benefits, as the industry would be in its infancy – not knowing the type, volume and value of products being brought to market.
B: Local Authorities / Port Health Authorities
Costs
Pre-market authorisation process
11.12 No additional costs incurred to Local Authorities and Port Health Authorities. The FSA will be responsible for pre-market authorisation process.
Public register of PBOs for food/f
11.13 No additional costs incurred to Local Authorities and Port Health Authorities. The FSA will be responsible for the public register.
Provisions for enforcement of requirements under new framework
11.14 Familiarisation cost (one-off) to Local Authorities: there will be familiarisation with changes to the provisions for enforcement of requirements under new framework. Whilst these changes relate to England, there are also indirect effects on the other countries of the UK. The cost of this has been calculated on the basis of the following estimates/assumptions:
- It would take one environmental health officer (EHO) per LA a total of 3 hours for familiarisation and dissemination;
- the median hourly rate of £25.69 (footnote 8) for an EHO;
- there are 313 LAs in England, 22 in Wales and 11 in NI that would need to familiarise themselves with the guidance.
11.15 To calculate the total cost, the hourly wage (£25.69) of an EHO is multiplied by the total familiarisation time (3 hours). This is then multiplied by the total number of LAs (346) giving a total one-off familiarisation cost of approximately £27,000. A breakdown of the cost by country is shown in Table 1 below:
Table 1: Familiarisation costs to LAs by country: England, Wales and Northern Ireland
| Country | Familiarisation cost |
|---|---|
|
England |
£24,000 |
|
Wales |
£1,700 |
|
Northern Ireland |
£850 |
|
Total |
£27,000 |
11.16 Familiarisation cost (one-off) to Port Health Authorities (PHA): there will be familiarisation with legislative changes under the new framework. Whilst these changes relate to England, there are also indirect effects on the other countries of the UK. The cost of this has been calculated on the basis of the following estimates/assumptions:
- it would take one inspector of standards and regulations (TSO) per PHA a total of 3 hours for familiarisation and dissemination;
- the median hourly rate of £19.40 (footnote 9) for a TSO;
- there are a total of 37 PHAs, 29 in England, 4 in Wales and 4 in NI that would need to familiarise themselves with the guidance.
11.17 To calculate the total cost, the hourly wage (£19.40) of a TSO is multiplied by the total familiarisation time (3 hours). This is then multiplied by the total number of PHAs (37) giving a total one-off familiarisation cost of approximately £2,200. A breakdown of the cost by country is shown in Table 2 below:
Table 2: Familiarisation costs to PHAs by country: England, Wales and Northern Ireland
| Country | Familiarisation cost |
|---|---|
|
England |
£1,700 |
|
Wales |
£230 |
|
Northern Ireland |
£230 |
|
Total |
£2,200 |
11.18 Cost to LAs of attending training (one-off): Although the training is provided by the FSA, there would be an opportunity cost to LAs when EHOs attend the training. The cost has been calculated on the basis of the following estimates/assumptions:
- all environmental health officers (EHO) will attend the training that will take 3 hours to complete;
- the median hourly rate of £25.69 (footnote 10) for an EHO
- there are 703 LA officers in England, 185 in Wales and 68 in Northern Ireland
11.19 To calculate the total cost, the hourly wage (£25.69) of an EHO is multiplied by the total training time (3 hours). This is then multiplied by the total number of LA officers (956) giving a total one-off cost of attending the training of approximately £74,000. A breakdown of the cost by country is shown in Table 3 below:
Table 3: Training costs to LAs by country: England, Wales and Northern Ireland
| Country | Training cost |
|---|---|
|
England |
£54,000 |
|
Wales |
£14,000 |
|
Northern Ireland |
£5,200 |
|
Total |
£74,000 |
11.20 Cost to PHAs of attending training (transition): Although the training is provided by the FSA, there would be an opportunity cost to PHAs when TSOs attend the training, hence an associated cost to PHAs of the time required and invested in receiving the training. It is assumed that all PHA TSOs would attend the training. However, a lack of data on the number of TSOs means that we are unable to quantify and monetise the total one-off cost to PHAs of attending the training.
General enforcement
11.21 No material ongoing costs to LA/PHAs identified. Monitoring and investigation of suspected non-compliance of PBOs for food/feed will fall under business as usual within the existing role for TSOs and EHOs in respect of food/feed law.
Benefits
11.22 No direct benefits to Local Authorities and Port Health Authorities from the change in legislation has been identified, however the public register is a tool that may help with the extra complexity of enforcement.
C: Consumers
Costs
11.23 No direct cost to consumers from the change in legislation has been identified.
Benefits
Public Register of PBOs for Food/Feed
11.24 The public register will improve transparency of the new regulatory regime. As FSA consumer research into transparency tells us, consumers want to be able to see that food is being regulated by an organisation they trust, and that open and transparent publication increases trust. A register will provide the visible regulation that consumers told us would support their confidence in precision bred food. The recommended register will provide information on the purposes of the genetic change, which consumers also told us they wanted to know to help them assess benefits and risks.
D: Wider impacts
11.25 The wider impacts of precision breeding itself have been considered and accounted for in DEFRA’s impact assessment (PDF). To avoid the risk of double counting, this consultation considers only the wider impacts of the proposed new regulatory framework.
Traditional breeders and organic sector
11.26 Traditional breeders and organic food and feed firms could be disproportionately impacted should they choose to continue to supply non-PBO food/feed. They are likely to face higher costs in trying to guarantee their products are non-PBO; as the genetic changes will be indistinguishable from traditionally bred counterparts there will be difficulty in detection and segregation would be necessary.
Competition and innovation
11.27 Although the new regulatory framework could facilitate the increase in private investment in Research and Development (R&D) in this new technology; there is a risk that, domestic producers could be affected by increased competition from countries where similar regulation is already established. This risk is particularly high in the short term, as UK adoption of PB technologies is in its infancy.
Consultation questions: Assessment of impact
We have carried out an assessment of the impact arising from our proposals.
a. Do you agree with the assumptions and estimates used to calculate one-off familiarisation costs to businesses? [Yes/No/Don’t know]
b. Do you agree with the assumptions and estimates used to calculate one-off familiarisation cost to Local Authorities in England, Wales and Northern Ireland? [Yes/No/Don’t know]
c. Do you agree with the assumptions and estimates used to calculate one-off training cost to Local Authorities in England? [yes/no/don’t know]
d. Do you agree with the impacts that the FSA has identified within this consultation? [Yes/No/Don’t know]
e. Are you aware of any impacts of the proposed new regulatory framework that the FSA has not identified in this consultation? [Yes/No]
f. Do you agree with the wider impacts identified in this consultation? [Yes/No/Don’t know]
g. Please explain your reasons for your position [Free text]
12. Responses
12. 1 Responses to this consultation are required by close on 8 January 2024. Please state, in your response, whether you are responding as a private individual or on behalf of an organisation/company (including details of any stakeholders your organisation represents).
12.2 Please respond preferably using the online consultation response form. Alternatively, you can respond via e-mail at: precisionbreeding@food.gov.uk
12.3 For information on how the FSA handles your personal data, please refer to the Consultation privacy notice.
13. Further information
13.1 If you require a more accessible format of this document please send details to the contact e-mail address for responses to this consultation above and your request will be considered.
13.2 This consultation has been prepared in accordance with HM Government consultation principles.
Thank you on behalf of the FSA for participating in this public consultation.
Precision Breeding Team
Food Standards Agency
November 2023
Annex A - Pre-market authorisation flowchart
Basic overview of notification and application process
This diagram outlines the FSA's process for the authorisation of precision bred organisms (PBOs) for food/feed in England.
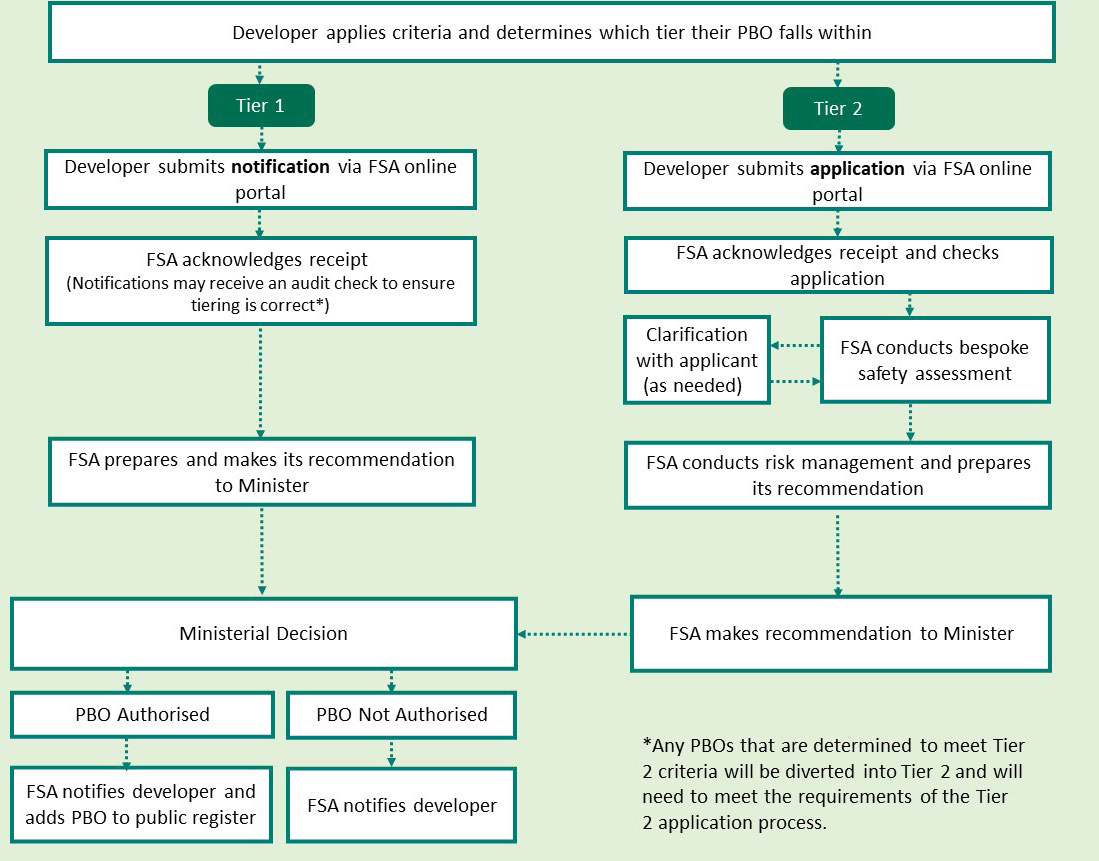
Transparency
The following information on PBOs for food/feed may be published online on food.gov or scientific advisory committee websites:
- summaries of valid Notifications / Applications: Placed on FSA Public Register of Regulated Product Applications (excludes commercially sensitive information)
- scientific Committee consideration: Papers and minutes of meetings (excludes commercially sensitive information)
- FSA Safety Assessments (excludes commercially sensitive information)
- information on authorised PBOs for food/feed: Placed on public Register (excludes commercially sensitive information)
Annex B - Glossary of terms
ACNFP – Advisory Committee on Novel Foods and Processes. An independent expert committee comprising of scientists and specialist experts from a wide range of scientific disciplines, who actively advise the FSA on matters pertaining to novel foods, traditional novel foods, food and feed products of genetic technologies and novel food processes including food irradiation.
Application - The legal process under which developers must apply to the FSA for a marketing authorisation for a PBO for food/feed that they have determined as a Tier 2 PBO.
Developer – Those developing PBOs for food/feed and submitting notifications (Tier 1 PBOs) or applications (Tier 2 PBOs) for pre-market authorisation.
Genetic modification (GM)- Process of altering the genome of a living thing. In England, this would only apply if it has been altered in a way that could not have occurred naturally. Genetic modification allows us to produce plants, animals and micro-organisms with specific qualities. Genetic modification allows just one individual gene, or a small number of genes, to be inserted into a plant or animal. GM may also stand for 'genetically modified'.
Genetically modified organism (GMO) – Plants and animals that have had their genome altered in a way that could not have occurred naturally or by mean of traditional breeding are known as GMOs.
Notification – The legal process under which developers must notify the FSA about a PBO for food/feed that they have determined as a Tier 1 PBO to seek a marketing authorisation.
Precision bred organism (PBO) – An organism that has had its genome edited using precision breeding techniques. Under the Precision Breeding Act, this is distinct from a genetically modified organism (GMO).
Precision breeding – Precision breeding refers to techniques, such as gene editing, used to make precise changes to the genome of plants or animals. These changes, which could also have been obtained by traditional breeding techniques, allow obtaining targeted beneficial changes. This is different to genetic modification (GM), which produces crops containing genetic changes that could not have occurred through traditional breeding or occur naturally.
Traditional breeding (methods) – Traditional breeding refers to techniques using a wide range of conservative tools or traditional processes to develop or improve desirable traits in plants or animals and select their presence in offspring. (Legal Reference: Section 1(6) concerning the PBO definition in the Genetic Technology (Precision Breeding) Act 2023).
Triage – The stage in the pre-market authorisation process at which a decision is taken based on scientific criteria as to whether a PBO for food/feed is a Tier 1 or Tier 2 PBO and hence whether a notification or an application is required.
-
Section 1 of the Act provides a definition of ‘precision bred organism’ for the purposes of the Act and secondary legislation made under it. Section 30(2) of the Act provides that, for the purposes of Part 3 "…food or feed is produced 'from' a precision bred organism if it contains, consists of, or is otherwise derived from, the precision bred organism."
-
Section 1 of the Act provides a definition of 'precision bred organism' for the purposes of the Act and secondary legislation made under it. As well as criteria for the classification of 'precision bred'.
-
Statement of (ACNFP) on Precision Bred Organisms (PBOs) - January 2023
-
Retained Regulation 178/2002 in respect of England, Wales and Scotland and Regulation (EC) No 178/2002 in respect of Northern Ireland.
-
Section 28(4)(b) of the Act stipulates that secondary legislation may not authorise an inspector to enter a private dwelling without the consent of the occupier except in exercise of a warrant issues by a Justice of the Peace
-
To ensure consistency in our calculations we have adopted the Standard Cost Model (SCM) approach published by BEIS. Where we have used wage rate data, we have taken hourly wage rates from the 2022 (provisional) Annual Survey of Hours and Earnings (ASHE), using the median rate of pay (SOC 241) and uplifting by 22% to account for overheads in line with RPC guidance note on implementation costs (pdf)
-
Impact Assessment - Genetic Technology (Precision Breeding) Bill (PDF) - Note, caveats of this estimate include; it is the membership of the British Society of Plant Breeders (BSPB) and not all commercial plant breeders are members (potentially underestimating the figure). Additionally, not all breeders may choose to develop PB plants (potentially overestimating the figure).
-
2022 (provisional) Annual Survey of Hours and Earnings (ASHE), using the median rate of pay (SOC 2483) and uplifting by 22% to account for overheads in line with RPC guidance note on implementation costs (PDF).
-
2022 (provisional) Annual Survey of Hours and Earnings (ASHE), using the median rate of pay (SOC 3581) and uplifting by 22% to account for overheads in line with RPC guidance note on implementation costs (PDF).
-
2022 (provisional) Annual Survey of Hours and Earnings (ASHE), using the median rate of pay (SOC 2483) and uplifting by 22% to account for overheads in line with RPC guidance note on implementation costs (PDF).
Revision log
Published: 8 November 2023
Last updated: 6 November 2024